
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 33P
Predict the major organic product from each of the following reaction sequences.
(a)
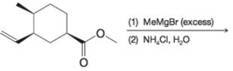
(b)
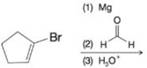
(c)
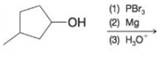
(d)
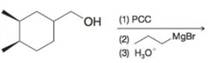
(e)
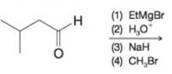
(f)
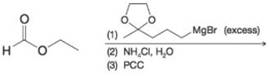
(g)
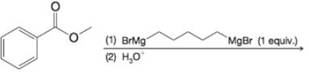
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw a structural formula for the alcohol formed by treating each alkene with borane
in tetrahydrofuran (THF) followed by hydrogen peroxide in aqueous sodium hydroxide,
and specify stereochemistry where appropriate.
(a)
(d)
(b)
(e)
(c)
(a)
(b)
(c)
Suggest a synthesis of the following alkene (A) using a Wittig reaction strategy. Draw
the starting material(s), key reagent and a full reaction mechanism including an
explanation of the observed geometry.
Which of the following (B) and (C) will favour the enol form? Briefly explain your
reasoning.
Predict the product(s) and provide a mechanism for each of the following
transformations:
(i)
(ii)
OMe
OMe
Base
OEt
NaOEt
(c) Answer each of the questions below that relate to acetophenone:
Xo
(i)
(ii)
(iii)
Draw the structure of the enol form of acetophenone.
Give a stepwise mechanism for the conversion of acetophenone into its
enol form.
Show how each of the three compounds A, B and C below can be
prepared from acetophenone. Explain clearly what reactants/reagents
would be required in each case.
odocor
A
B
Br
C
Chapter 12 Solutions
Organic Chemistry
Ch. 12 - Prob. 1PPCh. 12 - Prob. 2PPCh. 12 - Prob. 3PPCh. 12 - PRACTICE PROBLEM 12.4 Predict the products of the...Ch. 12 - Prob. 5PPCh. 12 - Prob. 6PPCh. 12 - Practice Problem 12.7
Provide retrosynthetic...Ch. 12 - Prob. 8PPCh. 12 - What products would you expect from the reaction...Ch. 12 - What products would you expect from the reaction...
Ch. 12 - What product (or products) would be formed from...Ch. 12 - Prob. 12PCh. 12 - 12.13 Write reaction conditions and the product...Ch. 12 - Prob. 14PCh. 12 - Predict the organic product from each of the...Ch. 12 - Predict the organic product from each of the...Ch. 12 - Predict the organic product from each of the...Ch. 12 - Predict the major organic product from each of the...Ch. 12 - Synthesize each of the following compounds from...Ch. 12 - Prob. 20PCh. 12 - 21. Write a mechanism for the following reaction....Ch. 12 - Prob. 22PCh. 12 - 23. What organic products A-H would you expect...Ch. 12 - Prob. 24PCh. 12 - Show how 1-pentanol could be transformed into each...Ch. 12 - Provide the reagents needed to accomplish...Ch. 12 - Prob. 27PCh. 12 - For each of the following alcohols, write a...Ch. 12 - Prob. 29PCh. 12 - Prob. 30PCh. 12 - Prob. 31PCh. 12 - Prob. 32PCh. 12 - Predict the major organic product from each of the...Ch. 12 - 34. Synthesize the following compound using...Ch. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - 37. Explain how and IR spectroscopy could be used...Ch. 12 - 38. An unknown X shows a broad absorption band in...Ch. 12 - Prob. 39PCh. 12 - The problem below is directed toward devising a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
In qualitative analysis, Ca2+ and Ba2+ are separated from Na+, K+, and Mg2+ by adding aqueous (NH4)2CO3 to a so...
General Chemistry: Atoms First
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Chlorine gas is to be heated front 120°C and 1 atm to 180°C. Calculate the heat input (kW) required to heat a s...
Elementary Principles of Chemical Processes, Binder Ready Version
Predict the major product in each of the following cases:
Organic Chemistry As a Second Language: Second Semester Topics
Q4. Which property of rubbing alcohol is a chemical property?
a) Density (0.786 g/cm3)
b) Flammability
c) Bo...
Chemistry: A Molecular Approach (4th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- . How would you carry out each of the following syntheses: ? (a) (b)arrow_forwardNucleophilic aromatic substitution provides one of the common methods for making phenols. ) Show how you would synthesize the following phenols, using benzene or toluene as your aromatic starting material, and explain why mixtures of products would be obtained in some cases. (a) m-cresol (b) p-n-butylphenolarrow_forwardGuiding your reasoning by retrosynthetic analysis, show how you could prepare each of the following compounds from the given starting material and any necessary organic or inorganic reagents. All require more than one synthetic step. (a) Cyclopentyl iodide from cyclopentane (b) 1-Bromo-2-methylpropane from 2-bromo-2-methylpropane (c) meso-2,3-Dibromobutane from 2-butyne (d) 1-Heptene from 1-bromopentane (e) cis-2-Hexene from 1,2-dibromopentane (f) Butyl methyl ether (CH3CH2CH2CH2OCH3) from 1-butenearrow_forward
- Propose a synthesis for each of the following compounds. (a) OH (b) (c)arrow_forwardIdentify ALL of the nucleophiles in each of the following molecules. H. (a) (c) NH2 tu (b) Li (d) NaOHarrow_forwardDescribe the characteristic infrared absorption frequencies that would allow you to distinguish between the following pairs of compounds. (a) cyclohex-2-enone and cyclohex-3-enone (b) cyclohexanol and cyclohexanonearrow_forward
- don't know Which of the following, when reacted with HCl, would result in the formation of the same major product at both low and high temperatures? (a) (b) (c) (d)arrow_forwardPredict the products when each compound undergo Ozonolysis (a) (b) (c) , (d) (e)arrow_forwarddon't know Which of the following, when reacted with HCl, would result in the formation of the same major product at both low and high temperatures? (a) (b)arrow_forward
- (a) What reagents would be used for the conversion of alkene A into the target? (b) What reaction is involved in the conversion of alcohol B into alkene A? Suggest a reagent that might affect this transformation. (c) Give a retrosynthetic analysis showing the disconnection of B, the synthons produced that lead to the synthetic equivalents given (draw their structures).arrow_forward(b) A student wanted to synthesize methyl tert-butyl ether. He attempted the synthesis by adding sodium methoxide to tert-butyl chloride, but he obtained none of the desired product (1) (ii) Use an equation to show the product formed in this reaction Propose a suitable William ether synthetic route for methyl tert-butyl ether tach l.arrow_forward9. (a) Provide the reagent necessary to carry out the following chemical transformations. (1) (ii)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY