
(a)
Interpretation: The molecular formulas of the compound represented by data points A, D, and E needs to be determined.
Concept Introduction: Empirical formula of a compound shows the simplest ratio of atoms present in the compound, on the other hand, the molecular formula shows the actual number of moles of atoms present in the compound.
(a)
Explanation of Solution
The empirical formula of the compound is given
Since the empirical formula is
Or,
Therefore, the molecular formula of the compound is
Now, from the given mass of carbon, the number of moles of carbon in the compound will be:
It confirms that there are 2 mol of carbon atoms in the compound. Thus, confirms the molecular formula equals to
Now, the molar mass corresponding point D is 150 g/mol. Using the empirical mass, the molecular formula can be determined as follows:
Or,
Therefore, the molecular formula of the compound is
Also, the point corresponding mass of carbon equals to 60 g. Now, from the given mass of carbon, the number of moles of carbon in the compound will be:
It confirms that there are 5 mol of carbon atoms in the compound. Thus, confirms the molecular formula equals to
Molar mass corresponding to point E is 180 g/mol. Using the empirical mass, the molecular formula can be determined as follows:
Or,
Therefore, the molecular formula of the compound is
Also, the point corresponds mass of carbon equals to 70 g. Now, from the given mass of carbon, the number of moles of carbon in the compound will be:
It confirms that there are 6 mol of carbon atoms in the compound. Thus, confirms the molecular formula equals to
Therefore, the molecular formula of compounds A, D, and E is
(b)
Interpretation: The slope of the line needs to be determined, and whether the value is consistent with the empirical formula or not needs to be determined.
Concept Introduction: An equation for a straight-line graph can be represented as follows:
Here, m is the slope of the graph and c is the intercept.
(b)
Explanation of Solution
Now, the given plot is a straight line; thus, the equation for the graph will be
Here, the intercept of the graph is zero; thus, the equation can be rewritten as follows:
Now, taking any one point say A, the molar mass of the compound is 60 g/mol, and the mass of the carbon atom is 25 g. Thus,
The value of m will be:
Thus, the slope of the graph is 2.5. Now, this is the ratio of the molar mass of the compound to the mass of carbon in the compound. From the slope, the mass percentage of carbon can be calculated as follows:
Yes, the value is constant with the empirical formula of the compound as the empirical formula is given
(c)
Interpretation: The value of x and y for the two other valid data points that fall on the same line between A and D needs to be determined.
Concept Introduction: From a graph, the new points on the line can be easily determined by checking drawing vertical and horizontal lines from that points to check where the values lie on the x and y-axis.
(c)
Explanation of Solution
From the graph, it is clear that the two points that fall on the same line between A and D will have the value of x and y as follows:
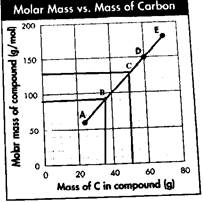
For B: The value of x, which is the mass of the carbon atom, will be 35 g, and the value of y, which is the molar mass of the compound, will be 90 g/mol. Therefore, the point on the graph will be B
For C: The value of x, which is the mass of the carbon atom, will be 50 g and the value of y, which is the molar mass of the compound, will be 130 g/mol. Therefore, the point on the graph will be C
Chapter 10 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





