
(a)
Interpretation: To determine the shape and structure of the given molecule.
Concept Introduction: The dots surrounding an element's symbol are a depiction of the valence electrons of that element, or electron dot structure. The valence electrons of the atom are equal to the number of dots in the electron dot structure.
(a)
Answer to Problem 122A
The shape is pyramidal and
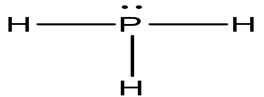
Explanation of Solution
There are 8 valence electrons in the PH3 Lewis structure. Just two valence electrons are required for hydrogen (H) to have a complete outer shell. Given that P and N are in the same group on the periodic table, the Lewis structure of PH3 and NH3 are similar.
Shape: Pyramidal
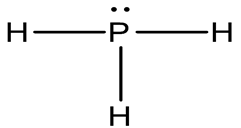
(b)
Interpretation: To determine the shape and structure of the given molecule.
Concept Introduction: The dots surrounding an element's symbol are a depiction of the valence electrons of that element, or electron dot structure. The valence electrons of the atom are equal to the number of dots in the electron dot structure.
(b)
Answer to Problem 122A
The shape is liner and
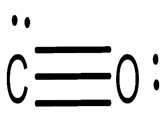
Explanation of Solution
One carbon atom, one oxygen atom, two pi bonds, and one sigma bond make up one molecule of carbon monoxide.
Shape: Linear
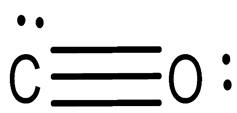
(c)
Interpretation: To determine the shape and structure of the given molecule.
Concept Introduction: The dots surrounding an element's symbol are a depiction of the valence electrons of that element, or electron dot structure. The valence electrons of the atom are equal to the number of dots in the electron dot structure.
(c)
Answer to Problem 122A
The shape is liner and
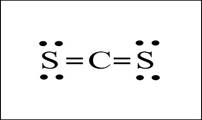
Explanation of Solution
The
Shape: Linear
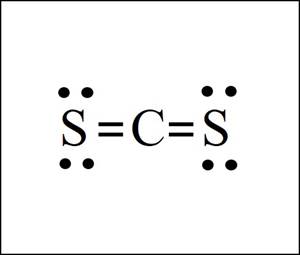
(d)
Interpretation: To determine the shape and structure of the given molecule.
Concept Introduction: The dots surrounding an element's symbol are a depiction of the valence electrons of that element, or electron dot structure. The valence electrons of the atom are equal to the number of dots in the electron dot structure.
(d)
Answer to Problem 122A
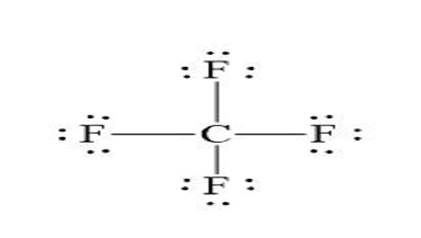
The shape is Tetrahedral and the structure is:
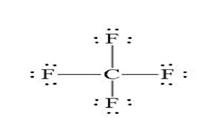
Explanation of Solution
The five standard steps for writing Lewis’s dot structures are used to derive the Lewis dot structure for
Shape: Tetrahedral
Chapter 10 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





