
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 63P
Draw in all the carbon and hydrogen atoms in each molecule.
a. 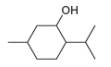 c.
c. 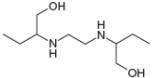
methanol ethambutol
(isolated from peppermint oil) (drug used to treat tuberculosis)
b. 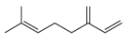 d.
d. 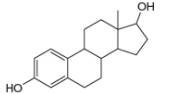
myrcene estradiol
(isolated from bayberry) (a female sex harmone)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Of the hydrocarbons given here, which contains the most carbon atoms?
a.
butane
c.
ethane
b.
pentane
d.
propane
Which of the following pairs is incorrect?
Select one:
a. ethane – C2H4
b. pentane – C5H12
c. hexane – C6H14
d. heptane – C7H16
e. octane – C8H18
When drawing the Lewis structure of an
organic compound with more than one
central atom, it is helpful to note:
A. Organic molecules are comprised of carbon atoms bonded to
each other.
B. Organic molecules are often comprised of mainly carbon and
hydrogen atoms.
C. Organic molecules often contain oxygen.
D. Both A and B.
E. A, B, and C.
Chapter 1 Solutions
Organic Chemistry (6th Edition)
Ch. 1.1 - While the most common isotope of nitrogen has a...Ch. 1.2 - Label each bond in the following compounds as...Ch. 1.3 - Draw a valid Lewis structure for each species. a....Ch. 1.3 - Prob. 9PCh. 1.4 - Draw Lewis structures for each molecular formula....Ch. 1.6 - Classify each pair of compounds as isomers or...Ch. 1.6 - Prob. 12PCh. 1.6 - Prob. 13PCh. 1.6 - Prob. 14PCh. 1.6 - Prob. 16P
Ch. 1.6 - Prob. 17PCh. 1.7 - Prob. 18PCh. 1.7 - Prob. 19PCh. 1.7 - Using the principles of VSEPR theory, you can...Ch. 1.8 - Convert each condensed formula to a Lewis...Ch. 1.8 - Prob. 22PCh. 1.8 - Prob. 23PCh. 1.8 - Convert each skeletal structure to a complete...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Assign formal charges to each and atom in the...Ch. 1 - Prob. 44PCh. 1 - Prob. 46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - Prob. 54PCh. 1 - Consider compounds A-D, which contain both a...Ch. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - 1.61 Convert each molecule into a skeletal...Ch. 1 - Prob. 65PCh. 1 - Predict the hybridization and geometry around each...Ch. 1 - Prob. 68PCh. 1 - Ketene, , is an unusual organic molecule that has...Ch. 1 - Rank the following bonds in order of increasing...Ch. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Prob. 74PCh. 1 - Anacin is an over-the-counter pain reliever that...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 83PCh. 1 - Prob. 84PCh. 1 - Prob. 85PCh. 1 - Prob. 86PCh. 1 - Prob. 87PCh. 1 - Prob. 88P
Additional Science Textbook Solutions
Find more solutions based on key concepts
The method to determine the volume of a powered solid, liquid and a rock needs to be determined. Concept introd...
Living by Chemistry
For each of the following 2-dimensional shapes, determine the highest order rotation axis of symmetry.
Inorganic Chemistry
Characterize each of the following structures as aromatic, nonaromatic, or antiaromatic:
Answer: _____
Organic Chemistry As a Second Language: Second Semester Topics
What is the pH range for acidic solutions? For basic solutions?
EBK INTRODUCTION TO CHEMISTRY
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, & Biological Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need answer for question 2.3 1. Draw three molecules of each compound, i.e. draw the molecules next to one another to visualize IMFa. Propane, CH3CH2CH3b. Heptane, CH3(CH2)5CH3 c. Propanol, CH3CH2CH2OH d. Heptanol, CH3(CH2)6OH2. For each compound, consider whether or not H-bonding can occur between its molecules. Use a dashed line to show any H-bonding.3. For each compound, consider whether or not any polar bonds are presenta. Use a different coloured pen to identify any polar bondsb. Which compounds are polar? Which compounds are nonpolar? Explain your reasoning4. Compare your drawings for propanol and heptanol.a. Which compound has stronger dispersion forces? Explain your answerb. Which compound has a higher boiling point? Explain your answer5. Compare your drawings of heptanes and heptanola. Which compound is more polar? Explain your answerb. Which compound is more soluble in water? Explain your answerarrow_forward2. Based on the MolView activity, which compound has the most covalent bonds? A. Water B. Hydrogen sulfide C. Carbon tetrachloride D. Methyl chloride E. Propanearrow_forwardConsider a molecule of methanimine (CH3N) compared to a molecule of methylamine (CH5N). The C-N bond in methanimine is than the C-N bond in methylamine. A. longer and stronger B. longer and weaker C. shorter and stronger D. shorter and weaker E. the same strength and length asarrow_forward
- 1. Consider serine (it is expected you will need to refer to a chart of the 20 common amino acids to find structure information for this and other amino acids). a. Draw its complete Lewis structure of serine (show all atoms, bonds and lone pairs). Draw the version of the structure without any charges b. Identify all of the functional groups C. Draw the Zwitterion form of serine d. Explain how the Zwitterion is formed from the uncharged versionarrow_forwardH₂C. C H₂ www CI CI Name using I.U.P.A.C nomenclature.arrow_forwardName the following aromatic compounds. a. b. CH3 OR methylbenzene с. d. ÇH3 ÇH3 .CH3 `CH3 е. f. ÇH3 CH3 H2C CH2 CH3 `CH3 g. h.arrow_forward
- A. Choose the letter of the best answer. 1. What is a saturated hydrocarbon? A. Any compound consisting of carbon and hydrogen only. B. Any compound consisting of carbon and hydrogen and oxygen only. C. Any compound consisting of carbon and hydrogen only, in which some of the carbon atoms are joined to each other by double or triple bonds. D. Any compound consisting of carbon and hydrogen only, in which all the carbon atoms are joined to each other by single bonds. 2. What kind of hydrocarbon is C3H14? A. alkane C. alkyne B. alkene D. alcohol H. H H. 3. This molecule is an example of an... H. A. alkane C. alkyne B. alkene D. alcohol 4. Which is an example of an ether? А. CН3ОН B. CH3CH2CH2CI C. CH3CH2OCH2CH3 D. CH3CH2COOCH3 5. What is the simplest organic compound? A. methane C. ethanol B. acetone D. ethanearrow_forward3. Name the following compounds (2 pts each) .F b. а.arrow_forward3. The functional group in compound below is called... (Please see attached image) A. ketone B. acid C. ester D. amine E. None of the others F. amidearrow_forward
- The chemical structure of glycine (C,H,NO,) is shown below. Highlight each atom that is in an amino group. H. H :0: 1. H -N- C -C –0 Harrow_forward1.63 Draw in all the carbon and hydrogen atoms in each molecule. OH OH HOH do H .N. а. b. С. d. N. myrcene menthol (isolated from bayberry) HO. Но (isolated from peppermint oil) ethambutol estradiol (drug used to treat tuberculosis) (a female sex hormone)arrow_forwardWhat type of molecule is each?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY