
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
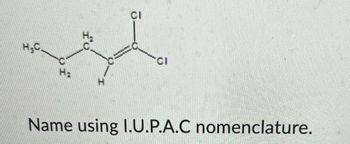
Transcribed Image Text:H₂C.
C
H₂
www
CI
CI
Name using I.U.P.A.C nomenclature.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- HBr HBr H₂SO4, H₂O HgSO4 H2SO4, H₂O HgSO4 1. BH3 2. H₂O₂, NaOHarrow_forward1. You have the tools to accomplish one of the early synthesis of a major alkaloid. Porantherine (molecule M) is the main alkaloid of the low woody shrub Poranthera corymbosa distributed in the southwest. The following questions pertain to the attached synthetic scheme (J. Am. Chem. Soc. 1974, 96, 6516). The are key steps that utilize an aldol-like mechanism to close three rings (A) What kind of transformation is the D → E step; show NO mechanism, but what was added to D? Hint: molecule E clearly has a terminal alkene and ketone, but what is the third functional group (it's not another alkene)? You've done the mechanism for making this functional group in chapter 20. (B) The E F transformation is rather elegant and it is promoted by an acid (toluenesulfonic acid). It involves formation of an enol (tautomerization) followed by protonation on the C=C bond in the ring to form a resonance- stabilized carbocation. Show the mechanism of this cyclization starting from the enol (i.e. double…arrow_forward1. Describe Joule's experiment and the conclusion he came up with as a result.arrow_forward
- CH. A. H-C-CH₂CH, CH₂ b. C. d. K. h OH CH₂ CH -OH OH Ch₂ bv 1. Br₂ 22 g LDA 2 1. MSCI 3.0 4. (CH₂)₂5 0 00, CHI DO CHO CH₂ X CH₂arrow_forward2. MeCN n-BuLi; Et₂C=O Me- Me OH CNarrow_forwardE M H3O+ Ph Br CrO3, H₂SO4, H₂O CH3MgBr B CH3CH₂CH₂MgBr F N R CH3Br Ph -CH3 Br с SOCI₂, G 1. (CH₂)SO, (COCI)₂ 2. N(CH₂CH3)3 K O NaH CH3CH₂CH₂Br S NaBH4 D H Ph P CH3 NaOCH3 PBr3 CH3OH, H₂SO4 T i> Ph 1. ? 2. ? HỌ CH,CH2CH3 + diastereomerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY