
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 73P
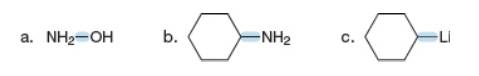
Use the symbols
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Compare the average N–O bond in your Lewis structures of NO2+ and NO2–. Based on the trends you identified above, which average N–O bond do you expect to be stronger? Which do you expect to be shorter? Explain your reasoning.
Stronger N–O Bond
NO2+
NO2–
Shorter N–O Bond
NO2+
NO2–
Arrange the highlighted bonds in the table below in decreasing order of polarity. That is, pick 1 for the most polar bond, pick 2 for the next most
polar bond, and so on.
bond
polarity
:F:
(Choose one)
(Choose one)
|(Choose one)
Use the symbols δ+ and δ- to indicate the polarity of the highlighted bonds.
Chapter 1 Solutions
Organic Chemistry (6th Edition)
Ch. 1.1 - While the most common isotope of nitrogen has a...Ch. 1.2 - Label each bond in the following compounds as...Ch. 1.3 - Draw a valid Lewis structure for each species. a....Ch. 1.3 - Prob. 9PCh. 1.4 - Draw Lewis structures for each molecular formula....Ch. 1.6 - Classify each pair of compounds as isomers or...Ch. 1.6 - Prob. 12PCh. 1.6 - Prob. 13PCh. 1.6 - Prob. 14PCh. 1.6 - Prob. 16P
Ch. 1.6 - Prob. 17PCh. 1.7 - Prob. 18PCh. 1.7 - Prob. 19PCh. 1.7 - Using the principles of VSEPR theory, you can...Ch. 1.8 - Convert each condensed formula to a Lewis...Ch. 1.8 - Prob. 22PCh. 1.8 - Prob. 23PCh. 1.8 - Convert each skeletal structure to a complete...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Assign formal charges to each and atom in the...Ch. 1 - Prob. 44PCh. 1 - Prob. 46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - Prob. 54PCh. 1 - Consider compounds A-D, which contain both a...Ch. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - 1.61 Convert each molecule into a skeletal...Ch. 1 - Prob. 65PCh. 1 - Predict the hybridization and geometry around each...Ch. 1 - Prob. 68PCh. 1 - Ketene, , is an unusual organic molecule that has...Ch. 1 - Rank the following bonds in order of increasing...Ch. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Prob. 74PCh. 1 - Anacin is an over-the-counter pain reliever that...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 83PCh. 1 - Prob. 84PCh. 1 - Prob. 85PCh. 1 - Prob. 86PCh. 1 - Prob. 87PCh. 1 - Prob. 88P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Bond polarities play an important role in determining the structure of proteins. Using the electronegativity values as shown, arrange the following covalent bonds—all commonly found in amino acids—in orderof increasing polarity. Then designate the positive and negative atoms using the symbols δ+ and δ–: C–H, C–N, C–O, N–H, O–H, S–Harrow_forwardRank the following bonds in order of increasing bond polarity: H−O, H−F, H−P, H−S.arrow_forwardExplain lectronegativity and Bond Polarity?arrow_forward
- (a) Use a polar arrow to indicate the polarity of each bond: N¬H, F¬N, I¬Cl. (b) Rank the following bonds in order of increasing polarity and decreasing percent ionic character: H¬N, H¬O, H¬C.arrow_forwardDescribe the differences between covalent bonding andionic bondingarrow_forwardWhat information can you use to predict whether a bond between two atoms is covalent or ionic?arrow_forward
- Many organic compounds belong to a category of molecules called "hydrocarbons", meaning that they only contain hydrogen and carbons. An example of a simple hydrocarbon is shown below. Considering both the VSEPR shape of the molecule and electronegativity values of the elements and state whether you expect this simple hydrocarbon to be polar or nonpolar. Explain your answer. нн H-C-C-H ннarrow_forwardMolecule Lewis Structure Central atom Shape of Bond Polarity Polarity of (can be done on separate paper) Molecule Molecule # bonded # lone atoms Pairs HCN Н-С C-N Cl4 CH3CI С-Н C-CI C-H CH20 C-O NH3arrow_forwardArrange the highlighted bonds in the table below in decreasing order of polarity. That is, pick 1 for the most polar bond, pick 2 for the next most polar bond, and so on.arrow_forward
- Match each of the following with its correct answer in the lewis structure for the CS, molecule, the number of unshared pairs of electrons on the central carbon is: The number of Tt bond (s) in the Lewis structure of NO- is An electron in the n= 6 level in hydrogen atomlemits a photon with a wavelength of 93.8 nm. To what energy level does the electron move The formal charge on nitrogen in the Lewis structure of NO+ is Ch The formal charge on oxygen in the Lewis structure of NO+ is Cho POCOPHONE SHOT ON POCOPHONE F1arrow_forwardPredict which bond in the following groups is the most ionic in character. Calculate ∆EN for each to check your predictions. H–Cl, H–Br, H–F Na–O, Li–O, K–Oarrow_forwardThe terms purely covalent and polar covalent are used to describe covalent bonds. Explain the difference between these two terms:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY