
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.8, Problem 24P
Convert each skeletal structure to a complete structure with all
a. 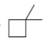 b.
b. 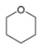 c.
c. 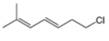 d.
d. 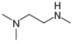
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Convert each skeletal structure to a complete structure with all C's, H's, and lone pairs drawn in.
Draw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge.
a. diethyl ether, (CH3CH2)2O, the rst general anesthetic used in medical proceduresb. acrylonitrile, CH2CHCN, starting material used to manufacture synthetic Orlon bersc. dihydroxyacetone, (HOCH2)2CO, an ingredient in sunless tanning productsd. acetic anhydride, (CH3CO)2O, a reagent used to synthesize aspirin
Draw the line bond or skeletal structure of the following condensed structures.
a. CH,CH,CH,CH,CH,CH,CH,
b. CH,CH,CH,CH,(CH,)CHCH,
c. (CH,),CHCH,CH,
d. CH,CH,CH,C(CH,),CH,CH,
Chapter 1 Solutions
Organic Chemistry (6th Edition)
Ch. 1.1 - While the most common isotope of nitrogen has a...Ch. 1.2 - Label each bond in the following compounds as...Ch. 1.3 - Draw a valid Lewis structure for each species. a....Ch. 1.3 - Prob. 9PCh. 1.4 - Draw Lewis structures for each molecular formula....Ch. 1.6 - Classify each pair of compounds as isomers or...Ch. 1.6 - Prob. 12PCh. 1.6 - Prob. 13PCh. 1.6 - Prob. 14PCh. 1.6 - Prob. 16P
Ch. 1.6 - Prob. 17PCh. 1.7 - Prob. 18PCh. 1.7 - Prob. 19PCh. 1.7 - Using the principles of VSEPR theory, you can...Ch. 1.8 - Convert each condensed formula to a Lewis...Ch. 1.8 - Prob. 22PCh. 1.8 - Prob. 23PCh. 1.8 - Convert each skeletal structure to a complete...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Assign formal charges to each and atom in the...Ch. 1 - Prob. 44PCh. 1 - Prob. 46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - Prob. 54PCh. 1 - Consider compounds A-D, which contain both a...Ch. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - 1.61 Convert each molecule into a skeletal...Ch. 1 - Prob. 65PCh. 1 - Predict the hybridization and geometry around each...Ch. 1 - Prob. 68PCh. 1 - Ketene, , is an unusual organic molecule that has...Ch. 1 - Rank the following bonds in order of increasing...Ch. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Prob. 74PCh. 1 - Anacin is an over-the-counter pain reliever that...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 83PCh. 1 - Prob. 84PCh. 1 - Prob. 85PCh. 1 - Prob. 86PCh. 1 - Prob. 87PCh. 1 - Prob. 88P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Convert the following condensed structures into skeletal structures: a. CH3CH2CH2CH2CH2CH2OH b. CH3CH2CH2CH2CH2CH3 c. CH3CH2CHCH2CHCH2CH3 d. CH3CH2CH2CH2OCH3 e. CH3CH2NHCH2CH2CH3 f. CH3CHCH2CH2CHCH3arrow_forwardConvert each shorthand structure to a complete structure with all atoms and lone pairs drawn in. a. (CH3)2CH(CH,),CH3 b. (CH3)3COH c. CH;CO2(CH2)3CH3 ÇI CI d. HO OCH(CH3)2arrow_forwardDraw in all hydrogens and lone pairs on the charged carbons in each ion. a. b. d.arrow_forward
- 2. Convert the following skeletal structures into condensed structures. a. b. C. d. - Iarrow_forwardDraw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge. a. diethyl ether, (CH;CH2);O, the first general anesthetic used in medical procedures b. acrylonitrile, CH;CHCN, starting material used to manufacture synthetic Orlon fibers c. dihydroxyacetone, (HOCH2)¿CO, an ingredient in sunless tanning products d. acetic anhydride, (CH;CO)20, a reagent used to synthesize aspirinarrow_forward12. Which bonding pattern is NOT typical of carbon atoms in organic compounds? A. B. C. D. E. 13. Which compound is classified as ether? A. CH3 -0- CH₁ B. C. D. CH3 O-H CH3-CH2-0-H CH3-CH2-0-CH3 E. CH–CH,NH,arrow_forward
- Draw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge. a diethyl ether, (CH3CH2)2O, the first general anesthetic used in medical procedures b. acrylonitrile, CH2CHCN, starting material used to manufacture synthetic Orlon fibers c.dihydroxyacetone, (HOCH2)2CO, an ingredient in sunless tanning products d.acetic anhydride, (CH3CO)2O, a reagent used to synthesize aspirinarrow_forwardConvert the following condensed structures into skeletal structures: a. CH3CH2CH2CH2CH2CH2OH b. CH3CH2CH2CH2OCH3 c. CH3CH2CH2CH2CH2CH3 d. CH3CH2NHCH2CH2CH3arrow_forwardConvert each skeletal structure to a complete structure with all C’s and H’s drawn in.arrow_forward
- Draw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge. a. diethyl ether, (CH3CH2)2O, the first general anesthetic used in medical procedures b.acrylonitrile, CH2CHCN, starting material used to manufacture synthetic Orlon fibers c.dihydroxyacetone, (HOCH2)2CO, an ingredient in sunless tanning products d.acetic anhydride, (CH3CO)2O, a reagent used to synthesize aspirinarrow_forwardConvert each skeletal structure to Kekulé structure. OH HO a. С. `N' menthol (isolated from peppermint oil) ethambutol (drug used to treat tuberculosis) OH b. d. myrcene (isolated from bayberry) HO estradiol (a female sex hormone) IZarrow_forward8. What is the correct skeletal structure for heptane? A. B. C. D. pound?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License