
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 67P
Predict the hybridization and geometry around each highlighted atom.
a. 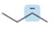 b.
b. 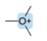 c.
c. 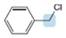 d.
d.  e.
e. 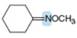
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
for each of the following compounds...
a. Provide the missing name
line structure
b. Build a model using the molecular model kit
c. Draw the lewis structure of the model and indicate the
hybridization of each ( atom.
or
5. Answer the following questions about the molecules CH2S (C is the centralatom).a. Draw the Lewis structure.b. Write the valence shell electron configuration for C.c. Draw the valence orbital diagram for C.d. Draw the hybridized orbital diagram for C.e. Give a bond description for each bond in the molecule.
1. Provide the hybridization for each of the indicated atoms
A
B
A.
A.
С.
C.
D.
2. Use partial charges(ô+, ô-) to indicate all polar bonds in the following molecules:
B.
B.
B.
Chapter 1 Solutions
Organic Chemistry (6th Edition)
Ch. 1.1 - While the most common isotope of nitrogen has a...Ch. 1.2 - Label each bond in the following compounds as...Ch. 1.3 - Draw a valid Lewis structure for each species. a....Ch. 1.3 - Prob. 9PCh. 1.4 - Draw Lewis structures for each molecular formula....Ch. 1.6 - Classify each pair of compounds as isomers or...Ch. 1.6 - Prob. 12PCh. 1.6 - Prob. 13PCh. 1.6 - Prob. 14PCh. 1.6 - Prob. 16P
Ch. 1.6 - Prob. 17PCh. 1.7 - Prob. 18PCh. 1.7 - Prob. 19PCh. 1.7 - Using the principles of VSEPR theory, you can...Ch. 1.8 - Convert each condensed formula to a Lewis...Ch. 1.8 - Prob. 22PCh. 1.8 - Prob. 23PCh. 1.8 - Convert each skeletal structure to a complete...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Assign formal charges to each and atom in the...Ch. 1 - Prob. 44PCh. 1 - Prob. 46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - Prob. 54PCh. 1 - Consider compounds A-D, which contain both a...Ch. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - 1.61 Convert each molecule into a skeletal...Ch. 1 - Prob. 65PCh. 1 - Predict the hybridization and geometry around each...Ch. 1 - Prob. 68PCh. 1 - Ketene, , is an unusual organic molecule that has...Ch. 1 - Rank the following bonds in order of increasing...Ch. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Prob. 74PCh. 1 - Anacin is an over-the-counter pain reliever that...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 83PCh. 1 - Prob. 84PCh. 1 - Prob. 85PCh. 1 - Prob. 86PCh. 1 - Prob. 87PCh. 1 - Prob. 88P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Number of Molecule valence electrons Formal Charge Electron-Group Geometry Molecular Geometry Resonance AIH2ATCI- Al: Al: Select one.. Al: Select one .. Select one ... AIHAT,Br Al: Al: Select one ... Al: Select one ... Select one ... FSIAS Si: Si: Select one .. Si: Select one ... Select one .. PHATCI P: P: Select one ... P: Select one.. Select one .. PCIFI P: P: Select one ... P: Select one ... Select one ... varrow_forwardNumber of Molecule valence electrons Formal Charge Electron-Group Geometry Molecular Geometry Resonance COSe C: C: Select one ... C: Select one.. Select one .. SH412 S: S: Select one .. S: Select one .. Select one ... SeHĄBr2 Se: Se: Select one... Se: Select one ... Select one ... AtGeN Ge: Ge: Select one ... Ge: Select one ... Select one ... CIGEP Ge: Ge: Select one... Ge: Select one ... Select one ... V 10000arrow_forwardPlease explain how to determine the correct answer choice in: Which of the following molecules or ions will exhibit delocalized bonding? NO2- NH4+ N3- a. NH4+ and N3- b. NO2- only c. NO2-, NH4+, and N3- d. N3- only e. NO2- and N3-arrow_forward
- Answer the following questions about octocrylene, a common sunscreen component. a. What is the hybridization of each C atom? b. How many lone pairs does octocrylene contain? c. What is the geometry around each C atom? d. Draw two additional resonance structures. e. Label all polar bonds.arrow_forwardAnswer the questions below about the highlighted atom in this Lewis structure: H. H. H C-C=C-H H. In how many sigma bonds does the highlighted atom participate? In how many pi bonds does the.highlighted atom participate? U What is the orbital hybridization of the highlighted atom?arrow_forwardNumber of Molecule valence electrons Formal Charge Electron-Group Geometry Molecular Geometry Resonance BH3B" B: B: Select one ... B: Select one ... Select one . SbAt2Br Sb: Sb: Select one ... Sb: Select one .. Select one BCI4 B: B: Select one.. B: Select one .. Select one .. V NBr3F* N: N: Select one.. N: Select one ... Select one . AIHCI3 Al: Al:Select one ... Al: Select one . Select one .arrow_forward
- PLS REFER TO THE PICTURE BELOW 1. How many covalent bonds are in the picture? 2. How mant type/s of covalent bond/s forming your double bond do you see? 3. What is/are the types of covalent bond/s forming your double bond do you see? 4. What type of hybrid orbital is the sigma bond? 5. Which electron configuration will you expect the highest energy needed to remove a valence electron?arrow_forwardConsider the following molecule: d. b. c. a. What is the hybridization at each of the labeled carbon atoms? b. What is the electron-pair geometry at each of the labeled carbon atoms? c. Which orbitals are responsible for the following bonds? Indicate which types of orbitals are used (i.e. s, p, sp?, etc.) and which types of bonds are formed (o or T). i. Bond a-b ii. Bond b-c iii. Bond d-e iv. Bond between carbon b and a hydrogen bound to it.arrow_forwardPlease note that "geometry" refers to the molecular or ionic geometry. a. What is the geometry of NO2 ? Geometry: b. What is the geometry of SO2 ? Geometry:arrow_forward
- For NOF, find: 1. # of valence electrons 2. Draw the Lewis Diagram 3. # of electron groups 4. # of lone pairs 5. electron group geometry drawing & name 6. molecular geometry drawing & name 7. bond angle(s)? 8. polar? 9. resonance?arrow_forwardSeCI4 1. Lewis dot structure? 2. Electron-pair geometry with vectors indicating bond dipole moments. Use dash and wedge bonds to indicate perspective. 3. Molecular structure with a vector indicating dipole moment. Use dash and wedge bonds to indicate perspective.arrow_forwardSchool Ad... ITATIONS OF ORGANIC MOLECULES Determining the electron group geometry and hybridization stat.. Answer the questions in the table about each labeled atom in the molecule below. a 0=c=N N- Atom Atom a Atom b Atom c What is the electron group geometry around each labeled atom? What is the hybridization of each labeled atom?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY