
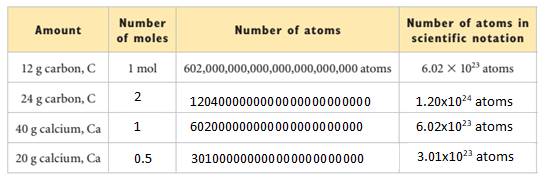
Interpretation: The given table needs to be completed.
Concept Introduction: The number of atoms in 1 mol of any substance is equal to
Explanation of Solution
Mass of 1 mol of carbon =12 g.
Number of moles of 24 g of C will be:
24 g C
Since, 1 mol C contains
So, 2 mol C contains
Similarly,
Mass of 1 mol of Ca=40 g.
Number of moles in 40 g of Ca will be:
So, 40 g Ca
Here,
1 mol Ca contains
Now,
20 g Ca
Also,
0.5 mol Ca contains
The complete table will be as follows:
Chapter U4 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Human Anatomy & Physiology (2nd Edition)
Chemistry: Structure and Properties (2nd Edition)
Microbiology: An Introduction
Campbell Essential Biology with Physiology (5th Edition)
Chemistry: The Central Science (14th Edition)
Introductory Chemistry (6th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





