
Concept explainers
(a)
Interpretation:The molecular view diagram of resulting mixture is to be drawn.
Concept introduction:The resulting solution formed by the mixing of two components having different chemical composition is known as mixture.The molecules are not uniformly distributed in water when slightly soluble compound is added to it.
(a)
Answer to Problem SV3RE
The molecular view diagram of resulting mixture is
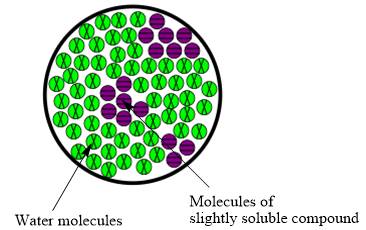
Explanation of Solution
When a slightly soluble compound is added to water only few molecules of slightly soluble compound comes in contact with water molecules. The bond between molecules of slightly soluble compound is not completely broken. The molecules form small patches in water.
(b)
Interpretation: The resulting mixture is homogeneous or heterogeneous is to be stated.
Concept introduction:The resulting solution formed by the mixing of two components having different chemical composition is known as mixture. The molecules are not uniformly distributed in water when slightly soluble compound is added to it.
(b)
Answer to Problem SV3RE
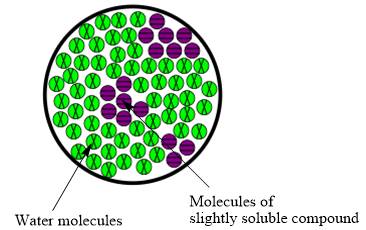
The resulting mixture of slightly soluble compound with water is heterogeneous mixture.
Explanation of Solution
The resulting solution formed by the mixing of two components having different chemical composition is known as mixture. When the molecules of solute are completely distributed in solvent then the mixture is classified as homogeneous mixture. However, when molecules are not uniformly distributed in solvent it is known as heterogeneous mixture. Therefore, the resulting mixture of slightly soluble compound with water is heterogeneous mixture.
Chapter U4 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Cosmic Perspective Fundamentals
Campbell Biology (11th Edition)
Campbell Essential Biology with Physiology (5th Edition)
College Physics: A Strategic Approach (3rd Edition)
Campbell Biology in Focus (2nd Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
- Show work with explanation. Don't give Ai generated solutionarrow_forwardDraw the structure of the acetal derived from 2,2-dimethyl-1,3-propanediol and butanal. Click and drag to start drawing a structure. X G Parrow_forwardPredict the major products of the following reaction. 田 Be sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. 口 + X C₁₂ Click and drag to start drawing a structure.arrow_forward
- Explain how the equation 4Fe(OH)2(s)+O2(g)→2Fe2O3(s)+4H2O(l) in the article illustrates the oxidation of the iron in the rectants.arrow_forwardIf you wanted to make something out of metal but didn't want it to rust, what are your options?arrow_forwardExplain how the equation 4Fe(OH)2(s) + O2(g)→2Fe2O3(s) + 4H2O(l) in the article illustrates the oxidation of the iron ions in the reactantsarrow_forward
- A Predict the major products of the following reaction. Be sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. + Cl₂ 2 X Click and drag to start drawing a structure.arrow_forwardC app.aktiv.com Predict reagents needed to complete this E2 elimination reaction. Br Problem 17 of 40 H3O+ A heat NaH B heat 0 D E (CH)COK heat CH₂ONa (CH)COH heat Donearrow_forwardPlease correct answer and don't use hand ratingarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





