
Concept explainers
(a)
Interpretation:
The mechanism for the given reaction has to be given.
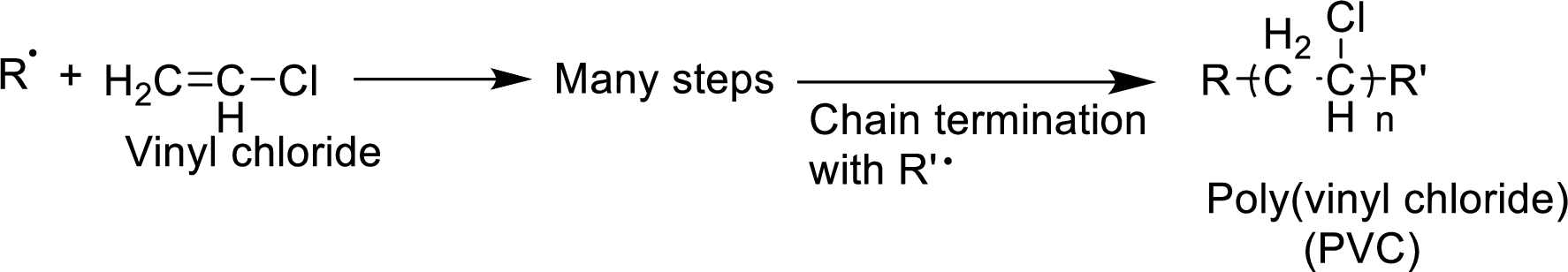
Concept Introduction:
The
- 1. Chain initiation
- 2. Chain propagation and
- 3. Chain termination.
Chain initiation occurs by the formation of radical from one of the monomer units. Propagation occurs by the reaction of the radicals with molecules. Chain termination occurs by neutralization of radicals.
(b)
Interpretation:
The mechanism for the formation of poly(styrene) from styrene has to be given and at which end of the styrene double bond, the
Concept Introduction:
The polymers are formed from the repetition monomer units. The polymerization process occurs in three steps.
- 1. Chain initiation
- 2. Chain propagation and
- 3. Chain termination.
Chain initiation occurs by the formation of radical from one of the monomer units. Propagation occurs by the reaction of the radicals with molecules. Chain termination occurs by neutralization of radicals.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry
- (A) The polymer known as polyvinyl acetate (PVAC) is used in paints and adhesives. Its structural formula is shown below. +CH;-CH¬ ... C=0 ČH3 1. Draw the structure formula of its monomer. 2. Write chemical equations that describe radical polymerization mechanism by which the PVAC could be prepared. 3. State whether head-to-tail or head-to-head orientation of monomer units in polymer chains predominates. Explain your answer by words and drawing.arrow_forward(4) Describe the synthesis of plastic especially polyethylene, 1,1- dichloroethane, and polyvinyl chloridearrow_forwardAcetylene is a very weak acid; however, it will react with moist silver(I) oxide and form water and a compound composed of silver and carbon. Addition of a solution of HCl to a 0.2352-g sample of the compound of silver andcarbon produced acetylene and 0.2822 g of AgCl.(a) What is the empirical formula of the compound of silver and carbon?(b) The production of acetylene on addition of HCl to the compound of silver and carbon suggests that the carbon is present as the acetylide ion, C22− . Write the formula of the compound showing the acetylide ion.arrow_forward
- (a) Describe the polymerization of a blend of maleic anhydride and 2-methyl-1,3- propanediol in the presence of an acid catalyst. Draw a chemical reaction scheme showing the intermediate and final products that are formed. Describe the reaction conditions that you would use to maximize the yield of polymer. (b) Draw the structure of the polymer, showing the repeat unit and end groups, that is obtained when the mole ratio of maleic anhydride to diol is 0.95 (c) What is the number average molecular weight of the polymer of Part (b) when the extent of reaction of maleic anhydride is (i) 90% and (ii) 100%. (d) Describe how the structure of the polymer is altered when it is further reacted with styrene in the presence of peroxide.arrow_forward(d) Propene can polymerize to form poly(propene). (i) State, with a reason, the atom economy for this reaction. (ii) Draw a section of this polymer, showing two repeat units.arrow_forwardConcerning the polymers produced from monomers containing a single C=C double bond, the following generalizations are apparent: (a) The polymers produced are almost always unsubstituted (i.e. ethylene) or have one substituent on the double bond or two substituents on the same carbon atom of the double bond. Monomers containing one or more substituents on each carbon of the double bond seldom polymerize. (b) Most chain polymerizations are carried out by radical initiation; relatively few are produced by ionic initiation. Why? Explain the reasons for these generalizationsarrow_forward
- Using each of the following polymers, (a) (b) ... (c) H H H H H C C C=0c=0c=0 H CH2 CH, CH2 H;C H3C H3C (i) Draw the condensed formula for the polymer. (ii) Propose a structure for the monomer from which the polymer was synthesized. I-arrow_forward1) (a) What is the difference between homopolymer and copolymer? (b) How can you classify the polymer depending on their skeletal structure?arrow_forwardShown below is the ε-caprolactone monomer where the biopolymer polycaprolactone (PCL) is synthesized from. (a) What is the functionality of the monomer? (b) What is polymerization mechanism to produce PCL? (c) Draw the repeating unit of the homopolymer given that the functional group is located at the terminal of the hydrocarbon chainarrow_forward
- 4. (a) (b) (c) (d) (e) (f) Draw the structure of the repeating unit in a polymer in which the monomer(s) is/are propylene 1,1-dichloroethylene vinyl acetate bisphenol A + phosgene ethylene glycol + terephthalic acid 1,4-chlorobenzene + sodium sulfide (a condensation polymerization affording NaCl)arrow_forward(a) What is the standard enthalpy of reaction for the reaction of 1.00 mol of propene with 1.00 mol of hydrogen to yield 1.00 mol of propane. (All gas phase) (b) What is the standard entropy change for the reaction? (c) What is the standard free energy change for the reaction carried out at 400 K? (You cannot use ∆Gfo data from the appendix). (d) Is the reaction spontaneous? (a) (b) (c) (d)arrow_forward(a) Hard contact lenses, which first became popular in the 1960s, were made by polymerizing methyl methacrylate [CH; =C(CH,)CO,CH3] to form poly(methyl methacrylate) (PMMA). Draw the structure of PMMA. (b) More comfortable softer contact lenses introduced in the 1970s were made by polymerizing hydroxyethyl methacrylate [CH2=C{CH)CO,CH,CH2OH] to form poly(hydroxyethyl methacrylate) (poly-HEMA). Draw the structure of poly-HEMA. Since neither polymer allows oxygen from the air to pass through to the retina, newer contact lenses that are both comfortable and oxygen-permeable have now been developed.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





