
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 8, Problem 8.12P
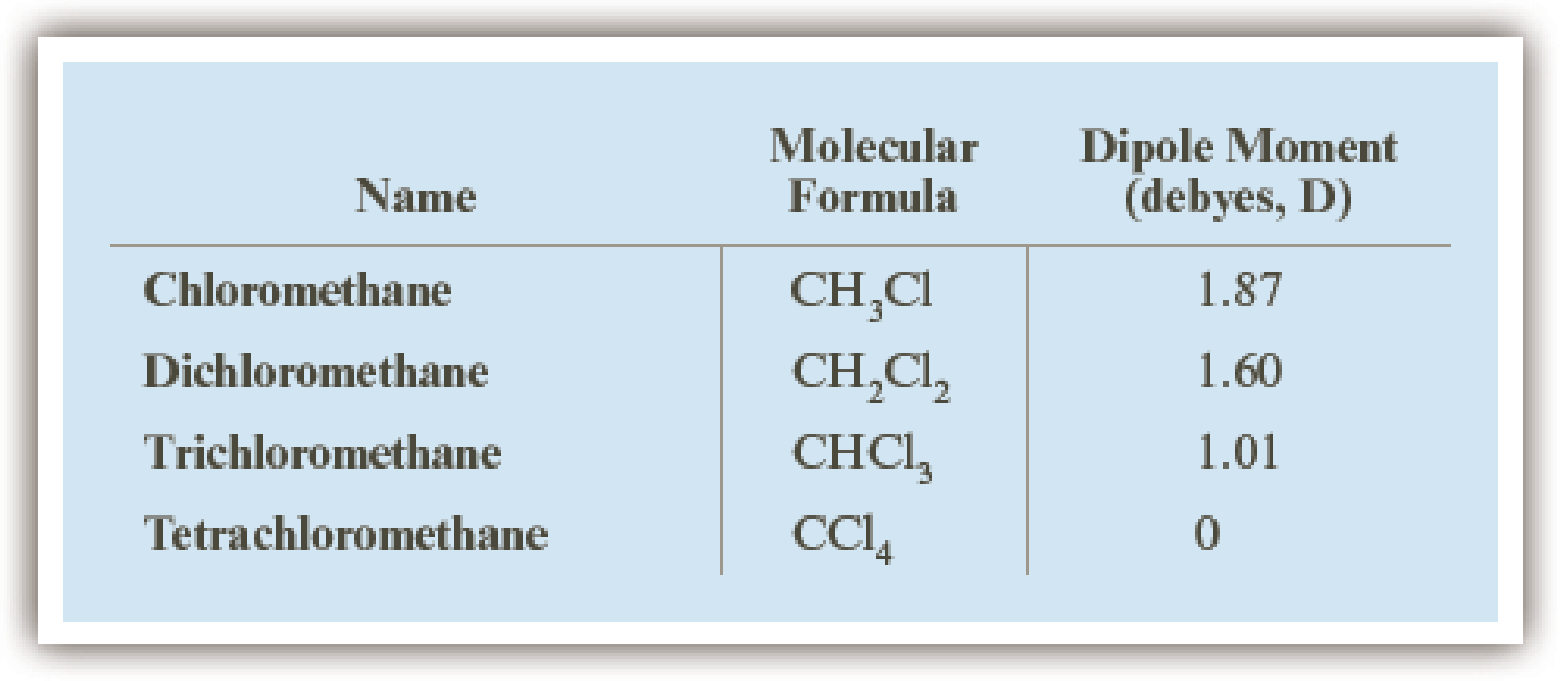
Account for the fact that among the chlorinated derivatives of methane, chloromethane has the largest dipole moment and tetrachloromethane has the smallest dipole moment.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
When bromine is added to two beakers, one containing phenyl isopropyl ether and the other containing cyclohexene, the bromine color in both beakers disappears. What observation could you make while performing this test that would allow you to distinguish the alkene from the aryl ether?
Organic Chemistry
Acid catalyzed dehydration reaction of 2-methyl-1-butanol produces 2-methyl-2-butene as the major product. Also acid catalyzed dehydration reaction of 3-methyl-1-butanol give the same product as major product. Explain the reason why both of the reaction produce the same product as the major product.
Explain the role strain plays on stability and reactivity of a cyclic alkane such as cyclopropane.
Chapter 8 Solutions
Organic Chemistry
Ch. 8.2 - Prob. 8.1PCh. 8.4 - Name and draw structural formulas for all...Ch. 8.4 - Using the table of bond dissociation enthalpies in...Ch. 8.5 - Prob. 8.4PCh. 8.6 - Given the solution to Example 8.5, predict the...Ch. 8.7 - Prob. 8.6PCh. 8.7 - Linoleic acid is shown below. What makes this...Ch. 8.7 - Prob. BQCh. 8.7 - Prob. CQCh. 8.7 - The strength of the HO bond in vitamin E is weaker...
Ch. 8.7 - Prob. EQCh. 8.8 - Prob. 8.7PCh. 8 - Prob. 8.8PCh. 8 - Prob. 8.9PCh. 8 - Prob. 8.10PCh. 8 - Prob. 8.11PCh. 8 - Account for the fact that among the chlorinated...Ch. 8 - Name and draw structural formulas for all possible...Ch. 8 - Prob. 8.14PCh. 8 - There are three constitutional isomers with the...Ch. 8 - Following is a balanced equation for bromination...Ch. 8 - Prob. 8.17PCh. 8 - Prob. 8.18PCh. 8 - Prob. 8.19PCh. 8 - Cyclobutane reacts with bromine to give...Ch. 8 - Prob. 8.21PCh. 8 - Following is a balanced equation for the allylic...Ch. 8 - Prob. 8.23PCh. 8 - Prob. 8.24PCh. 8 - The major product formed when methylenecyclohexane...Ch. 8 - Prob. 8.26PCh. 8 - Prob. 8.27PCh. 8 - Prob. 8.28PCh. 8 - Write the products of the following sequences of...Ch. 8 - Using your reaction roadmap as a guide, show...Ch. 8 - Prob. 8.31PCh. 8 - Prob. 8.32PCh. 8 - Prob. 8.33PCh. 8 - Prob. 8.34P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Compounds X and Y both have the formula C7H₁4. Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methylhexane. The heat of hydrogenation of X is greater than that of Y. Both X and Y react with HCI to give the same single C7H15Cl compound as the major product. What is the structure of X? • In cases where there is more than one answer, just draw one. 23 ▾ Sn [F ChemDoodleⓇ 146arrow_forward2-chloropropane is a major product of the reaction of chlorine with propane under ultraviolet light. Write the mechanism for this reaction including the initiation step and the two propagation steps.arrow_forwardWhen 2-chloropropane treated with NaOH and 1-chloropropane treated with NaOH separately produce two different functional groups. Provide both reactions and explain the two different functional groups produced.arrow_forward
- Bromine is a larger atom than chlorine, but the equilibrium constants in Table 3.9 indicate that a chloro substituent has a greater preference for the equatorial position than does a bromo substituent. Suggest an explanation for this fact.arrow_forwardWrite structural formulas for all ketones with the molecular formula C6H12O and give each its IUPAC name. Which of these ketones are chiral?arrow_forwardCompounds X and Y are stereoisomers having the formula C6H12.Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form hexane, and they each react with HBr to give a single bromoalkane product.Draw structural formulas for both X and Y.arrow_forward
- Ethanol, C2H5OH, and propane, C3H8, have approximately the same molar mass, yet ethanol has a much higher boiling point. Briefly explain why. Ethanol, C2H5OH, and dimethyl ether, CH3OCH3, have the same molar mass, yet ethanol has a much higher boiling point. Briefly explain why. Write an equation to show the reaction between ethanol, C2H5OH and methyllithium, CH3Li. Draw all non-bonding electrons and show electron flow with curved arrows. 37. Write an equation that shows the reaction between acetic acid (CH3COOH) and triethylamine (CH3CH2)3N. Draw all non-bonding lone electron pairs and show the electron flow with curved arrows.arrow_forwardWrite structural formulas for all aldehydes with the molecular formula C6H12O and give each its IUPAC name. Which of these aldehydes are chiralarrow_forwardWrite the structure of the following compounds 5-methyloctane-2,6-diol 2-methylpropane-1,2,3-triol 4-methylhexa-1-en-2,5-diol (2-methyl)hexyl phenyl ether decane-2,4,6,8-tetraol butylpropyl sulfide Dicyclopentyl ether Cyclobutylphenyl ether 2-chlorophenol Cyclopentyl epoxidearrow_forward
- Write a conformational structure for 1,2,3-trimethylcyclohexane in which all the methyl groups are axial and then show its more stable conformation.arrow_forwardCompound L undergoes dehydrohalogenation to form hydrogen bromide and 1-methylcyclohexene. What is dehydrohalogenation? State the condition required for dehydrohalogenation. Draw the structural formula of compound L and name L.arrow_forwardFrom studies of the dipole moment of 1,2-dichloroethane in the gas phase at room temperature (25°C), it is estimated that the ratio of molecules in the anti conformation to gauche conformation is 7.6 to 1. Calculate the difference in Gibbs free energy between these two conformations.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY