
Concept explainers
(a)
Interpretation:
Major product of the given reaction has to be identified.
Concept introduction:
Non-markonikov addition of
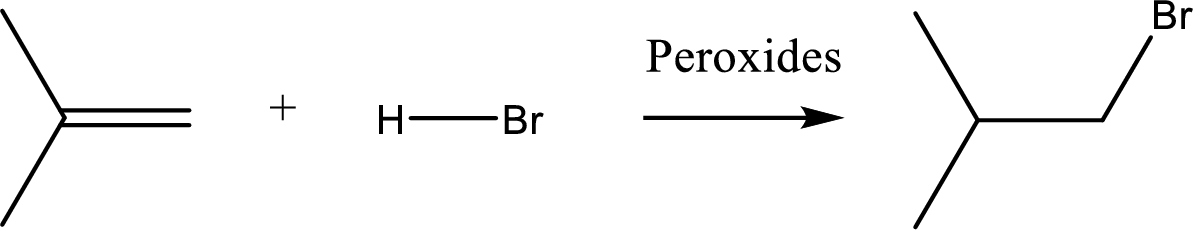
(b)
Interpretation:
Major product of the given reaction has to be identified.
Concept introduction:
Non-markonikov addition of
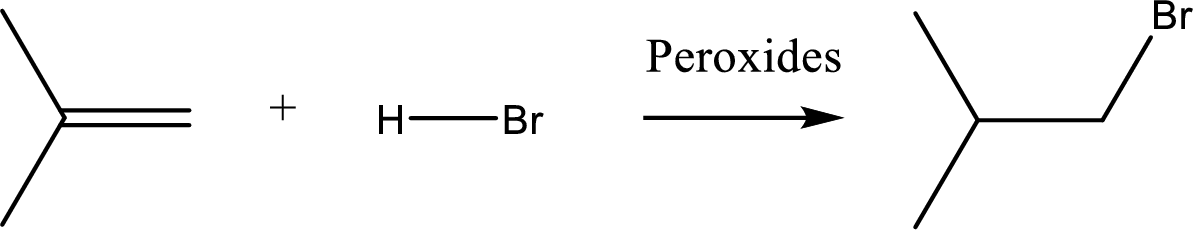
Trending nowThis is a popular solution!

Chapter 8 Solutions
Organic Chemistry
- Answer ALL parts. (a) Treatment of enone C with tetrabutylammonium fluoride leads to the formation of heterocycle D. 2 steps BU4NF C9H1602 A + B D OSIME,tBu (i) Enone C can be prepared from alkyne A and aldehyde B in two steps via a gold-catalysed reaction. Identify A and B and provide reagents/conditions for a synthesis of C. (ii) Give the structure of D and classify the ring-closing reaction for its formation from C according to Baldwin's rules (iii) Sketch the key molecular orbitals involved in the ring-closing reaction in part (ii) and hence explain whether the reaction is favourable or unfavourable.arrow_forwardWrite the structure of the major organic product formed in the reaction of 1-pentene with each of the following: (a) Hydrogen chloride (b) Dilute sulfuric acid (c) Diborane in diglyme, followed by basic hydrogen peroxide (d) Bromine in carbon tetrachloride (e) Bromine in water (f) Peroxyacetic acid (g) Ozone (h) Product of part (g) treated with zinc and water (i) Product of part (g) treated with dimethyl sulfide (CH3)2Sarrow_forwardGive IUPAC names for the following structures. (If appropriate, specify relative stereochemistry.) (a) (b) S Sarrow_forward
- (c) Arrange the following compounds in order of increasing acidity, and explain the reasons fo your choice of order: phenol, cyclohexanol, 2-fluorocyclohexanol, 2-fluoronhenolarrow_forwardGive the structure of the product formed on reaction of ethyl acetoacetate with each of the following: (a) 1-Bromopentane and sodium ethoxide (b) Saponification (basic hydrolysis) and decarboxylation of the product in part (a) (c) Methyl iodide and the product in part (a) treated with sodium ethoxide (d) Saponification and decarboxylation of the product in part (c) (e) 1-Bromo-3-chloropropane and one equivalent of sodium ethoxide (f) Product in part (e) treated with a second equivalent of sodium ethoxide (g) Saponification and decarboxylation of the product in part (f) (h) Phenyl vinyl ketone and sodium ethoxide (i) Saponification and decarboxylation of the product in part (h)arrow_forward(a) What reagents would be used for the conversion of alkene A into the target? (b) What reaction is involved in the conversion of alcohol B into alkene A? Suggest a reagent that might affect this transformation. (c) Give a retrosynthetic analysis showing the disconnection of B, the synthons produced that lead to the synthetic equivalents given (draw their structures).arrow_forward
- Show how you would synthesize the following compounds, starting with benzene or toluene and any necessary acyclic reagents. Assume para is the major product (and separable from ortho) in ortho, para mixtures.(a) ethoxybenzene (b) 1,2-dichloro-4-nitrobenzene (c) 1-phenylpropan-2-olarrow_forward11:43 Q1. (a) (c) (d) (b) Two stereoisomers of but-2-ene are formed when 2-bromobutane reacts with ethanolic potassium hydroxide. (i) Explain what is meant by the term stereoisomers. Library Name and outline a mechanism for the reaction of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH3)2C=CH₂ Name of mechanism Mechanism (ii) Draw the structures and give the names of the two stereoisomers of but-2-ene. Stereoisomer 1 Name (iii) Name this type of stereoisomerism. Select Name Stereoisomer 2 When 2-bromo-2-methylpropane reacts with aqueous potassium hydroxide, 2-methylpropan-2-ol is formed as shown by the following equation. CH3 H₂C-C-CH3 + KOH Br Page 2 of 14 CH3 H3C-C-CH3 + KBr ОН State the role of the hydroxide ions in this reaction. Write an equation for the reaction that occurs when CH3CH₂CH₂CH₂Br reacts with an excess of ammonia. Name the organic product of this reaction. Equation Name of product 9,284 Photos, 1,166 Videos For You…arrow_forwardShow how you would synthesize the following compounds, starting with benzene or toluene and any necessary acyclic reagents. Assume para is the major product (and separable from ortho) in ortho, para mixtures. (a) 3-nitro-4-bromobenzoic acid (b) 3-nitro-5-bromobenzoic acid (c) 4-butylphenol (d) 2-(4-methylphenyl)butan-2-olarrow_forward
- (b) A student wanted to synthesize methyl tert-butyl ether. He attempted the synthesis by adding sodium methoxide to tert-butyl chloride, but he obtained none of the desired product (1) (ii) Use an equation to show the product formed in this reaction Propose a suitable William ether synthetic route for methyl tert-butyl ether tach l.arrow_forward(a) Identify the major product of the following reaction. (b) Explain why the other product is not the major product.arrow_forwardConsider the tetracyclic compound with rings labeled A–D. (a) Which ring is the most reactive in electrophilic aromatic substitution? (b) Which ring is the least reactive in electrophilic aromatic substitution?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





