
Study Guide for Campbell Biology
11th Edition
ISBN: 9780134443775
Author: Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Jane B. Reece, Martha R. Taylor, Michael A. Pollock
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 2IQ
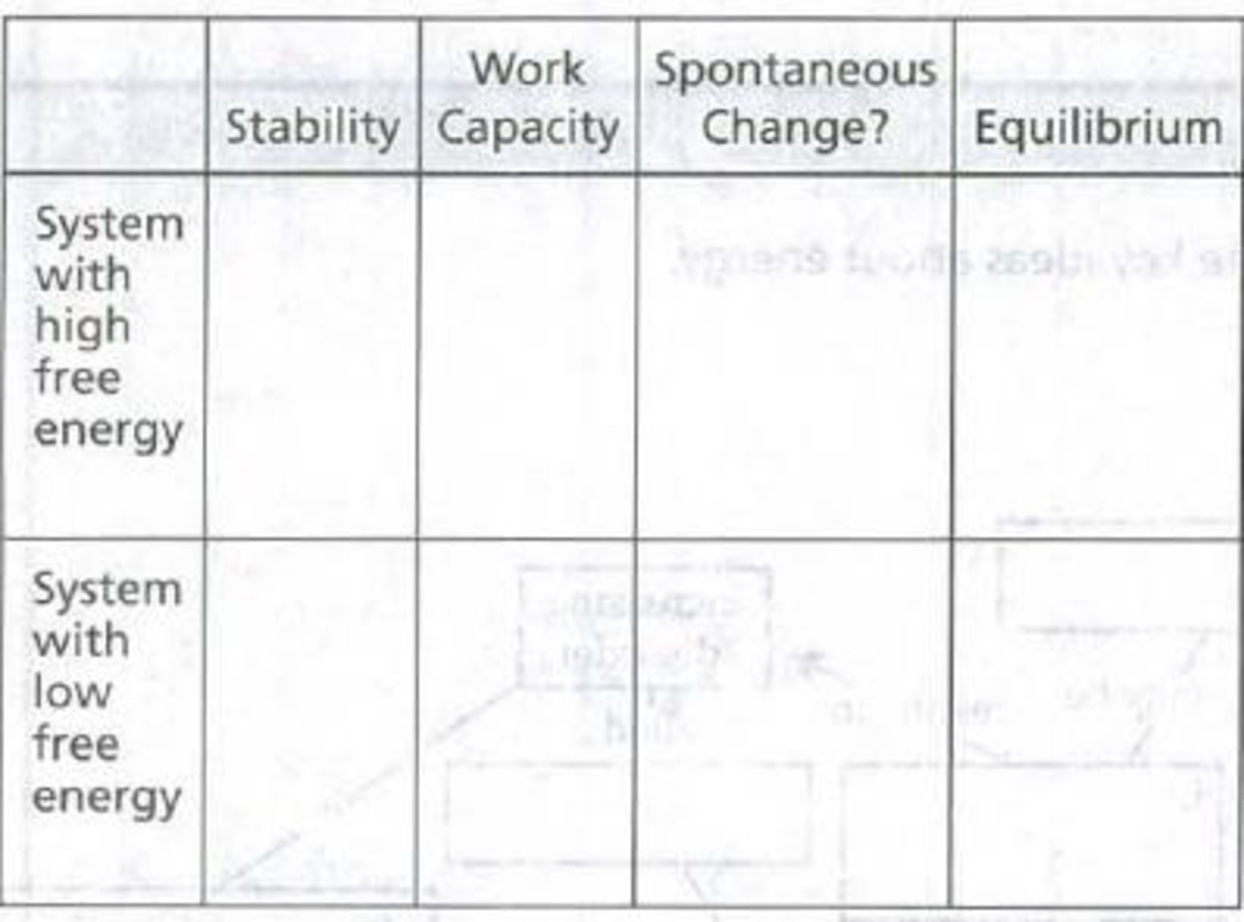
Complete the following table to indicate how the free energy of a system (or a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The molecule can absorb heat from the
environment without changing its chemical
structure, as will occur when there is a local
temperature increase. The molecule will have a
higher energy. If the increase in energy is above
KT (i.e., the entire environment has not
increased its temperature), the molecule will
come to thermal equilibrium with the
environment around it, and return to its original
energy state. Which one of the following is
example to this?
Select one:
a. The absorbance of radiant energy by
Melanin pigment in the skin.
b. Conversion of 11-cis form of retinal to
the all-trans form of retinal upon exposure
to light, with a maximum absorbance at 500
nm.
c. Thymine-thymine and thymine-cytosine
pyrimidine bridges formation upon
exposure of DNA to UV-B radiation.
d. The translucent to opaque conversion of
egg whites when cooked by increasing the
temperature.
Using collision theory, indicate which of the following statements regarding physical nature of the reactants is true:
a) physical nature of reactants
The physical nature of reactants does not influence the rate of reaction because reactions happen only on the molecular level.
Solids, liquids or gases all influence the rate of reaction the same because they are all in the physical state.
Solid state reactants react faster if the particle sizes are larger because reactions occur at the boundary surface with direct contact.
Gaseous-state reactions are faster than liquid-state or solid-state reactions because collisions between reactants are more frequent.
Use the following graph to diagram the energetics of a chemical reaction, with and without an enzyme. Be sure to position reactants and products at appropriate points and to indicate the stages in the reaction and the energy levels.
Chapter 8 Solutions
Study Guide for Campbell Biology
Ch. 8 - Complete the following concept map that summarizes...Ch. 8 - Complete the following table to indicate how the...Ch. 8 - Develop a concept map on free energy and G. The...Ch. 8 - Prob. 4IQCh. 8 - Prob. 5IQCh. 8 - In the following graph of an exergonic reaction...Ch. 8 - In the following diagram of a catalytic cycle,...Ch. 8 - Return to the diagram in Interactive Question 3.7,...Ch. 8 - Both ATP and ADP serve as regulators of enzyme...Ch. 8 - What is the relationship between the concept of...
Ch. 8 - What role do enzymes play in metabolism?Ch. 8 - ________ the totality of an organisms chemical...Ch. 8 - _______ pathways that use energy to synthesize...Ch. 8 - Prob. 3TYKFCh. 8 - _______ the most random form of energyCh. 8 - _______ term for the measure of disorder or...Ch. 8 - Prob. 6TYKFCh. 8 - _______ inhibitors that decrease an enzymes...Ch. 8 - Prob. 8TYKFCh. 8 - Prob. 9TYKFCh. 8 - Prob. 10TYKFCh. 8 - Catabolic and anabolic pathways are often coupled...Ch. 8 - Which statement most closely reflects the first...Ch. 8 - When a cell breaks down glucose, only about 34% of...Ch. 8 - Prob. 4TYKCh. 8 - Prob. 5TYKCh. 8 - Prob. 6TYKCh. 8 - One way in which a cell maintains metabolic...Ch. 8 - Prob. 8TYKCh. 8 - Prob. 9TYKCh. 8 - What is meant by an induced fit? a. The binding of...Ch. 8 - In an experiment, changing the pH from 7 to 6...Ch. 8 - Prob. 12TYKCh. 8 - Penicillin binds to the active site of an enzyme...Ch. 8 - Prob. 14TYKCh. 8 - Prob. 15TYKCh. 8 - Which line in the diagram indicates the G of the...Ch. 8 - Prob. 17TYKCh. 8 - Prob. 18TYKCh. 8 - Prob. 19TYKCh. 8 - Prob. 20TYK
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Here is a chemical reaction: H₂O2 -> O2 + H₂O What is/are the reactants? What is/are the products? H₂O2 is the product while O₂ and H₂O are reactants. There are no products or reactants as an enzyme is needed for the reaction to occur. There are no products or reactants as O2 and H₂O occur in the environment. O H₂O2 is the reactant while O₂ and H₂O are productsarrow_forwardCompare the energy dynamics of a reaction at equilibrium with the dynamics of a reaction not at equilibrium.arrow_forwardIn a transition state diagram, which of the following are features of the transition state (TS)? There may be more than one correct answer, select all that apply. The change in energy in ground state to the transition state represents the Gibbs Free Energy If the reaction is reversible, the TS will only progress forward to form products The TS occupies a trough The TS is associated with the highest energy The TS occupies the highest peakarrow_forward
- Define Kinetic energy and Potential energy.arrow_forwardIdentify the letter (A, B, C) that indicates the change in the energy of the entire reaction according to this energy profile. Explain why you choose the particular energy change. Include in your answer information about the ones you did not chose. alyzed Reaction Energy Exothermic Reaction Profilearrow_forwardDefine kinetic energy and potential energy. Is chemical energy kinetic or potential?arrow_forward
- Despite the thermal stability of covalent bonds in physiological systems, some of these bonds are sensitive to energy input from external sources. The molecule can absorb heat from the environment without changing its chemical structure, as will occur when there is a local temperature increase. If the increase in energy is above kT (i.e., the entire environment has not increased its temperature), the molecule will come to thermal equilibrium with the environment around it, and return to its original energy state. Which one of the following physiological process is example to this? Select one : a. Absorbance of light by 11-cis-retinal. b. Translucent to opaque conversion of egg whites after cooking. C. Absorbance of light by melanın. d. a & b e. a & c f.b&c g. a, b & carrow_forwardName and describe the four weak chemical interaction that occurs in biomolecules.arrow_forwardUse the following graph to diagram the energetics of a chemicalreaction, with and without an enzyme. Be sure to position reactantsand products at appropriate points and to indicate the stages in thereaction and the energy levels.arrow_forward
- What is the most common form of kinetic energy that is released from chemical energy in chemical reactions?arrow_forwardList the four factors that influence the rate of a chemical reaction and state whether increasing the factor will increase or decrease the rate of the reaction.arrow_forwardwhat is free Gibbs energy and write the expression of free energy change. Define the exergonic and endergonic processes.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning

Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning
Microbial Nutrition and Growth; Author: Scientist Cindy;https://www.youtube.com/watch?v=rK3UkyWjkl8;License: Standard YouTube License, CC-BY