
Concept explainers
(a)
Interpretation: The systematic name of the given organic compound is to be interpreted.
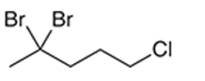
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of the chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a functional group is indicated by the secondary suffix.
(b)
Interpretation: The systematic name of the given organic compound is to be interpreted.
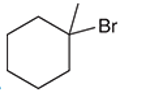
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a functional group is indicated by the secondary suffix.
(c)
Interpretation: The systematic name of the given organic compound is to be interpreted.
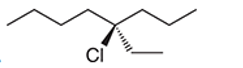
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a functional group is indicated by the secondary suffix.
(d)
Interpretation: The systematic name of the given organic compound is to be interpreted.
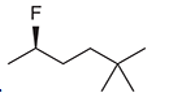
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a functional group is indicated by the secondary suffix.
(e)
Interpretation: The systematic name of the given organic compound is to be interpreted.
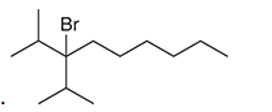
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a functional group is indicated by the secondary suffix.
(f)
Interpretation: The systematic name of the given organic compound is to be interpreted.
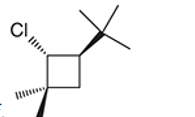
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The primary suffix in the root word indicates the presence of the single, double and triple bond in the molecule whereas the secondary suffix indicates the presence of a functional group in the molecule.
(g)
Interpretation: The systematic name of the given organic compound is to be interpreted.
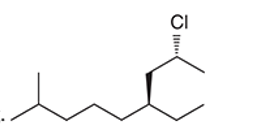
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a functional group is indicated by the secondary suffix.
(h)
Interpretation: The systematic name of the given organic compound is to be interpreted.
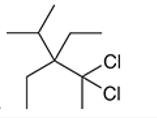
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a functional group is indicated by the secondary suffix.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Assign R or S designation to each of the following compoundsarrow_forwardDraw and name compounds that meet the following descriptions a.Three acid chlorides having the formula C6H9ClO b. Three amides having the formula C7H11NOarrow_forwardGive IUPAC names for the following compounds: b. c. e. f.arrow_forward
- Give both IUPAC names and common names for the following compounds. (CH3)2CHCHBrCOOHarrow_forwardGive IUPAC names for the following substances (red = O, blue = N):arrow_forward14-54 (Chemical Connections 14C) What is the color of dichromate ion, Cr2O72-? What is the color of chromium!Ill I ion, Cr’*? Explain how the conversion of one to the other is used in breath- alcohol screening.arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





