
(a)
Interpretation: The transition state of the given reaction is to be interpreted for the given conversion:
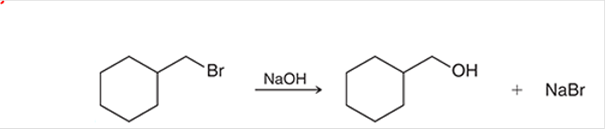
Concept introduction:
(b)
Interpretation: The transition state of the given reaction is to be interpreted for the given conversion:
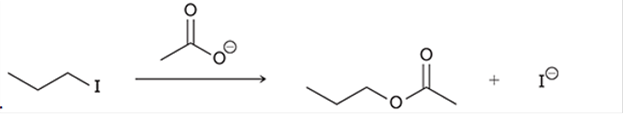
Concept introduction:
Haloalkanes can show the nucleophilic substitution reactions. There are two possible mechanisms
(c)
Interpretation: The transition state of the given reaction is to be interpreted for the given conversion:
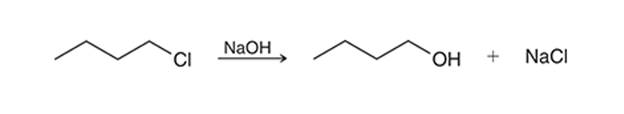
Concept introduction:
Haloalkanes can show the nucleophilic substitution reactions. There are two possible mechanisms
(d)
Interpretation: The transition state of the given reaction is to be interpreted for the given conversion:
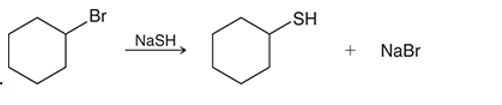
Concept introduction:
Haloalkanes can show the nucleophilic substitution reactions. There are two possible mechanisms
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Of the following, which represents the intermediate that is most likely formed in the first step of the mechanism? Br ОН Br ОН ОН ОН OH2arrow_forwardWhich arrow best describes the mechanism for the reaction? B C D O A 台灣券 O B OD 2. SI O-0arrow_forwardDescribe the mechanism of the following reaction. 1) H2N NH2 (excess) SH SH `CO2H AcO 2) КОН 3) H +arrow_forward
- Please draw the mechanism for the following reaction. Which compound given below most closely matches the intermediate formed in the first step of the mechanism. Reaction 1 D F میدهد H OH H HBr OH DCM E m OH H OHarrow_forward03 AllC :Draw the Mechanism (stepwise) of any ONE of the following reactions Ag(I) 1. OH H,SO4 NH 2. I B ENG 4) G T-T/-E/-arrow_forward7B What is the product of the following cleavage reaction and what is its stereochemistry? Me H Br H NaOEtarrow_forward
- Please draw the mechanism for the following reaction. Which compound given below most closely matches the intermediate formed in the first step of the mechanism. Reaction 1 HBr DCM A В C H D H E F G H, H. но. H :0:arrow_forward(3R, 4R)-3-Bromo-4-phenylhexane reacts with t-butoxide ion, but the major product has potential stereoisomers. The question is which stereoisomer predominates as the major product? Using scratch paper, draw the starting material and reagent, and then use what you know about reaction mechanisms to determine the correct mechanism for this reaction. Then, identify the product and the correct stereoisomer of the final product (R, S, E, or Z). Fill in the blanks below with the necessary information. a. What mechanism is controlling this reaction (SN1, SN2, E1, or E2)? b. Which stereoisomer product predominates (R, S, E, or Z)? c. What is the full name of the major product?arrow_forwardWrite the mechanism for the following reaction: `Br ELOH Write the mechanism and predict if the reaction above be faster or slower than the following reaction? Explain. Br ELOHarrow_forward
- On the lowest energy pathway to form the product of the following reaction, which species is a plausible transition state? (Dotted lines represent bonds in the process of forming or breaking.) x● HBr H---Br Br-H H----Br ठ Br-H productarrow_forwardWhich is the most thermodynamically stable intermediate in the reaction below? hvarrow_forwardDraw a representation of the transition state for the following endergonic mechanistic step. H,C-CH=CH, H-OH, H3C–CH-CH3 H,0arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





