
Concept explainers
Give IUPAC names for the following substances (red = O, blue = N):
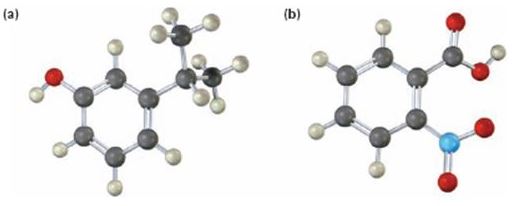
a)
Interpretation:
The IUPAC name of the compound shown is to be given.
Concept introduction:
Compounds with a hydroxyl group attached to the benzene ring are named as derivatives of phenol. Substituted phenols are named using the prefixes ortho (o), meta (m) and para (p). An ortho-substituted phenol has an another substituent in a 1,2-relationship on the ring. A meta-disubstituted phenol has an another substituent in a 1,3-relationship on the ring. A para-disubstituted phenol has another substituent in a 1,4-relationship on the ring.
To give:
The IUPAC name of the compound shown.
Answer to Problem 13VC
The IUPAC name of the compound is m-isopropylphenol.
Explanation of Solution
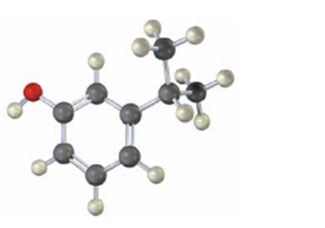
The compound shown is
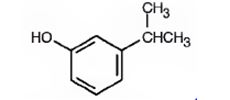
It has a hydroxyl and an isopropyl groups in the benzene ring in 1,3-relationship. Therefore its IUPAC name is m-isopropylphenol.
The IUPAC name of the compound is m-isopropylphenol.
b)
Interpretation:
The IUPAC name of the compound shown is to be given.
Concept introduction:
Compounds with a carboxyl group attached to the benzene ring are named as derivatives of benzoic acid. Substituted benzoic acids are named using the prefixes ortho (o), meta (m) and para (p). An ortho-substituted benzoic acid has an another substituent in a 1,2-relationship on the ring. A meta-disubstituted benzoic acid has an another substituent in a 1,3-relationship on the ring. A para-disubstituted benzoic acid has another substituent in a 1,4-relationship on the ring.
To give:
The IUPAC name of the compound shown.
Answer to Problem 13VC
The IUPAC name of the compound is o-nitrobenzoic acid.
Explanation of Solution
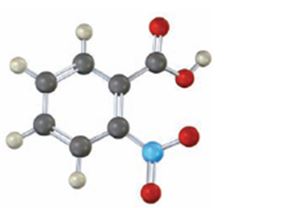
The compound shown is
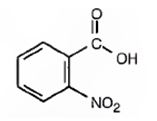
It has a carboxyl group and a nitro group in the benzene ring in 1,2-relationship. Therefore its IUPAC name is o-nitrobenzoic acid.
The IUPAC name of the compound is o-nitrobenzoic acid.
Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry
- 3B: Convert the starting material into the chiral epoxytriol below. OH OH = OH OHarrow_forward3D: Convert the aromatic triketone to the 1,3,5-triethylcyclohexane shown below. ہوئےarrow_forwardIndicate how to find the energy difference between two levels in cm-1, knowing that its value is 2.5x10-25 joules.arrow_forward
- The gyromagnetic ratio (gamma) for 1H is 2.675x108 s-1 T-1. If the applied field is 1,409 T what will be the separation between nuclear energy levels?arrow_forwardChances Ad ~stract one 11. (10pts total) Consider the radical chlorination of 1,3-diethylcyclohexane depicted below. 4 • 6H total $4th total Statistical pro 21 total 2 H A 2H 래 • 4H totul < 3°C-H werkest bund - abstraction he leads to then mo fac a) (6pts) How many unique mono-chlorinated products can be formed and what are the structures for the thermodynamically and statistically favored products? рос 6 -વા J Number of Unique Mono-Chlorinated Products Thermodynamically Favored Product Statistically Favored Product b) (4pts) Draw the arrow pushing mechanism for the FIRST propagation step (p-1) for the formation of the thermodynamically favored product. Only draw the p-1 step. You do not need to include lone pairs of electrons. No enthalpy calculation necessary H H-Clarrow_forwardWhat is the lone pair or charge that surrounds the nitrogen here to give it that negative charge?arrow_forward
- Last Name, Firs Statifically more chances to abstract one of these 6H 11. (10pts total) Consider the radical chlorination of 1,3-diethylcyclohexane depicted below. 4 • 6H total $ 4th total 21 total 4H total ZH 2H Statistical H < 3°C-H werkst - product bund abstraction here leads to the mo favored a) (6pts) How many unique mono-chlorinated products can be formed and what are the structures for the thermodynamically and statistically favored products? Proclict 6 Number of Unique Mono-Chlorinated Products f Thermodynamically Favored Product Statistically Favored Product b) (4pts) Draw the arrow pushing mechanism for the FIRST propagation step (p-1) for the formation of the thermodynamically favored product. Only draw the p-1 step. You do not need to include lone pairs of electrons. No enthalpy calculation necessary 'H H-Cl Waterfoxarrow_forward2. (a) Many main group oxides form acidic solutions when added to water. For example solid tetraphosphorous decaoxide reacts with water to produce phosphoric acid. Write a balanced chemical equation for this reaction. (b) Calcium phosphate reacts with silicon dioxide and carbon graphite at elevated temperatures to produce white phosphorous (P4) as a gas along with calcium silicate (Silcate ion is SiO3²-) and carbon monoxide. Write a balanced chemical equation for this reaction.arrow_forwardI find the solution way too brief and unsatisfactory as it does not clearly explain the solution provided in the problem.arrow_forward
- Please correct answer and don't used hand raitingarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardthis is an organic chemistry question please answer accordindly!! please post the solution in your hand writing not an AI generated answer please draw the figures and structures if needed to support your explanation hand drawn only!!!! answer the question in a very simple and straight forward manner thanks!!!!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



