
CHEMISTRY-TEXT
8th Edition
ISBN: 9780134856230
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 7.93SP
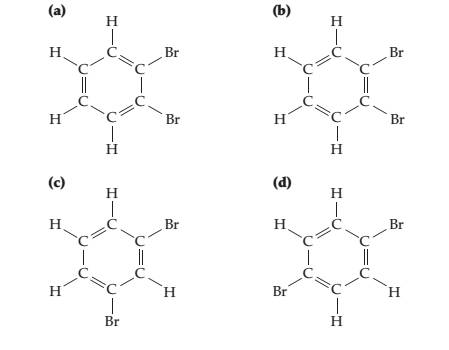
Four different structures (a), (b), (c), and (d) can be drawn for compounds named dibromobenzene, but only three different compounds actually exist. Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider the theoretical molecule KrCl3‾.
(a) Draw a valid Lewis structure for KrCl3‾. Show all lone pairs and use lines for bonds. Label all non-zeroformal charges on individual atoms and show the overall charge, if it exists, using square brackets.(b) What would you expect to be the molecular geometry for this ion? Fully explain your thought process,including all details about how successive lone pairs should be positioned within this electron geometry.(c) Draw this ion in 3-D, using hashed and wedged bonds as appropriate. Do not worry about labeling the overall or formal charge.
Given the pair of compounds,AlCl3 and CaCl2, which is more ionic and why ?
3. The following are some molecules:H2, HF, CO2, H2O, Cl2, NH3, CH4, CHCl3
(a) Which of the above molecules do/does not contain polar bond? Explain your answer.
(b) Which covalent bond has the highest bond polarity among the eight molecules?
(c) (i) Which of the above molecules are non-polar molecules?
(ii) Explain why these molecules are non-polar. 4. (d) Draw a diagram to illustrate the formation of hydrogen bonds in H2O.
Chapter 7 Solutions
CHEMISTRY-TEXT
Ch. 7 - Use the electro negativity values in Figure 7.4...Ch. 7 - Conceptual APPLY 7.2 An electrostatic potential...Ch. 7 - The dipole moment of AgCI in the gas phaseis...Ch. 7 - Predict which bond has greater percent ionic...Ch. 7 - Select the correct electron-dot structure for H2S...Ch. 7 - Use the octet rule to predict the molecular...Ch. 7 - Identify the correct electron-dot structure for...Ch. 7 - Draw an electron-dot structure for each of the...Ch. 7 - Select the correct electron-dot structure for...Ch. 7 - Identify the correct electron-dot structure(s) for...
Ch. 7 - Prob. 7.11PCh. 7 - Which oxygen species do you predict to be most...Ch. 7 - Draw an electron-dot structure for the following...Ch. 7 - There are two molecules with the formula C2H6O...Ch. 7 - The following structure is a representation of...Ch. 7 - Draw two possible electron-dot structures for the...Ch. 7 - Called “laughing gas, nitrous oxide (N2O) is...Ch. 7 - Draw as many resonance structures as possible for...Ch. 7 - Prob. 7.19PCh. 7 - Prob. 7.20ACh. 7 - Calculate the formal charge on each atom in the...Ch. 7 - Start with the electron-dot structure for the...Ch. 7 - Calculate formal charges on the C and O atoms in...Ch. 7 - Three resonance structures for anisole (Problem...Ch. 7 - The toxicity of the organophosphate insecticides...Ch. 7 - Prob. 7.26PCh. 7 - Prob. 7.27PCh. 7 - The following structure is a representation of the...Ch. 7 - The electron-dot structure for the nerve a gentsar...Ch. 7 - Draw the new electron-dot structures indicated by...Ch. 7 - The following diagram shows the potential energy...Ch. 7 - The following diagram shows the potential energy...Ch. 7 - Two electrostatic potential maps are shown, one of...Ch. 7 - Prob. 7.34CPCh. 7 - Which of the following drawings is most likely to...Ch. 7 - The following ball-and-stick molecular model is a...Ch. 7 - The following hall-and-stick molecular model is a...Ch. 7 - Sinapaldehyde, a compound present in the toasted...Ch. 7 - Vitamin C (ascorbic acid) has the following...Ch. 7 - Match the following descriptions with the type of...Ch. 7 - Why do two atoms come together to form a covalent...Ch. 7 - Explain the difference in the bond dissociation...Ch. 7 - Explain the difference in the bond dissociation...Ch. 7 - Predict which of the following bonds should be...Ch. 7 - Prob. 7.45SPCh. 7 - What general trends in electro negativity occur in...Ch. 7 - Predict the electro negativity of the undiscovered...Ch. 7 - Order the following elements according to...Ch. 7 - Order the following elements according to...Ch. 7 - Which of the following substances contain bonds...Ch. 7 - Use the electro negativity data in Figure 7.4 to...Ch. 7 - Show the direction of polarity for each of the...Ch. 7 - Show the direction of polarity for each of the...Ch. 7 - Which of the substances...Ch. 7 - Which of the substances...Ch. 7 - Order the following compounds according to the...Ch. 7 - Order the following compounds according to the...Ch. 7 - Prob. 7.58SPCh. 7 - Using only the elements Ca, Cl, and Si, give...Ch. 7 - The dipole moment of BrCl is 0.518 D, and the...Ch. 7 - Prob. 7.61SPCh. 7 - Prob. 7.62SPCh. 7 - Prob. 7.63SPCh. 7 - Why does the octet rule apply primarily to...Ch. 7 - Which of the following substances contains an atom...Ch. 7 - Draw electron-dot structures for the following...Ch. 7 - Draw electron-dot structures for the following...Ch. 7 - Identify the correct electron-dot structure for...Ch. 7 - Draw an electron.dot structure for the hydronium...Ch. 7 - Oxalic acid, H2C2O4 , is a mildly poisonous...Ch. 7 - Draw an electron-dot structure for carbon...Ch. 7 - Prob. 7.72SPCh. 7 - Identify the fourth-row elements, X, that form the...Ch. 7 - Write electron-dot structures for molecules with...Ch. 7 - Write electron-dot structures for molecules with...Ch. 7 - Which compound do you expect to have the stronger...Ch. 7 - Which compound do you expect to have the stronger...Ch. 7 - Draw an electron-dot structure for each of the...Ch. 7 - Prob. 7.79SPCh. 7 - Methylphenidate (C14H19NO2) , marketed as Ritalin,...Ch. 7 - Pregabalin (C8H17NO2) , marketed as Lyric a, is an...Ch. 7 - The following molecular model is that of...Ch. 7 - Ibuprofen C 13 H 18 O 2 marketed under such brand...Ch. 7 - Draw as many resonance structures as you can that...Ch. 7 - Draw as many resonance structures as you can for...Ch. 7 - Which of the following pairs of structures...Ch. 7 - Which of the following pairs of structures...Ch. 7 - Draw as many resonance structures as you can that...Ch. 7 - Draw as many resonance structures as you can that...Ch. 7 - Benzene has the following structural formula. Use...Ch. 7 - Draw three resonance structures for sulfur...Ch. 7 - Some mothballs used when storing clothes are made...Ch. 7 - Four different structures (a), (b), (c), and (d)...Ch. 7 - Draw an electron-dot structure for carbon...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Calculate formal charges for the C and O atoms in...Ch. 7 - Draw two electron-dot resonance structures that...Ch. 7 - Draw two electron-dot resonance structures that...Ch. 7 - Prob. 7.102SPCh. 7 - Prob. 7.103SPCh. 7 - Boron trifluoride reacts with dimethyl ether to...Ch. 7 - Thiofulminic acid, HCNS, has recently been...Ch. 7 - Draw two rcsonancc strutur for methyl isocyanate,...Ch. 7 - In the cyanatc ion. OCN , carbon is the central...Ch. 7 - Prob. 7.108MPCh. 7 - Prob. 7.109MPCh. 7 - Prob. 7.110MPCh. 7 - The neutral OH molecule has been implicated in...Ch. 7 - Prob. 7.112MPCh. 7 - Prob. 7.113MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- b) 'Rust' is the common name for Fe2O3. Write out the chemical name for this ionic compound using the appropriate roman numeral formalism.arrow_forwardWhich statement is false? and and and represent different molecules -- and X represent the same molecule Hot HO Хон represent the same molecule represent the same moleculearrow_forward1) Write formulas for compounds with the following names; Disulfur diiodide, Iodine monochloride, and Nitrogen monoxide.arrow_forward
- You are given a list of binary compounds (only two different elements) and asked to determine the relative melting points. d) Which would you expect to have a higher melting point, CHF, or CF,.. Why? e) Why would you expect both He and O, to have low melting points? Which one would have the higher melting point and why?arrow_forwardExplain the below Statement ? "Organic chemistry is the chemistry of compounds that contain theelement carbon"arrow_forwardLet E be any representative element. Following the patterns in the table, write formulas for the hydrogen and oxygen compounds of the following elements. Answers should be in the form of E2O (for E2O). (a) Sb Compound with hydrogen: Compound with oxygen: (b) Se Compound with hydrogen: Compound with oxygen: (c) Cl Compound with hydrogen: Compound with oxygen: (d) C Compound with hydrogen: Compound with oxygen:arrow_forward
- Problem Name the ionic compound formed from the following pairs of elements: (a) magnesium and nitrogen; (b) iodine and cadmium; (c) strontium and fluorine; (d) sulfur and cesium.Plan The key to naming a binary ionic compound is to recognize which element is the metal and which is the nonmetal. When in doubt, check the periodic table. We place the cation name first, add the suffix -ide to the nonmetal root, and place the anion name last.arrow_forwardWhich ionic compound is expected to form from combiningthe following pairs of elements? (a) barium and fluorine,(b) cesium and chlorine, (c) lithium and nitrogen, (d) aluminumand oxygen.arrow_forwardWhat are the chemical formulas for ( a ) disulfur dioxide and ( b ) iodine pentafluoride ?arrow_forward
- 1.Draw the electronic diagrams for the following compounds (showing outermost shell electrons only). (a) A compound formed from calcium and sulphur (b) a compound formed from aluminium and oxygen (c) A compound formed from nitrogen and chlorine (d) carbon dioxidearrow_forwardDoes the following compound exist?arrow_forward1) a) Identify the bonds formed between the following pairs of atoms as either covalent or ionic: zinc and fluoride, lithium and chloride, cesium and iodine, carbon and oxygen, chlorine and chlorine. b) What are likely formulas for the following molecules? Express your answer as a chemical formula. CH2Cl? BH? H?S SiBr?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT  World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY