
CHEMISTRY-TEXT
8th Edition
ISBN: 9780134856230
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 7.83SP
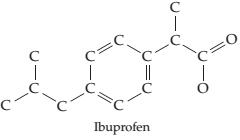
Ibuprofen
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Two important industrial chemicals, ethene, C2H4, and propene, C3H6, are produced by the steam (or thermal) cracking process:2C3 H8(g) ⟶ C2 H4(g) + C3 H6(g) + CH4(g) + H2(g)For each of the four carbon compounds, do the following:(a) Draw a Lewis structure.(b) Predict the geometry about the carbon atom.(c) Determine the hybridization of each type of carbon atom.
Following is a structural formula of the prescription drug famotidine, marketed by McNeil Consumer Pharmaceuticals Co. under the name Pepcid. The primary clinical use of Pepcid is for the treatment of active duodenal ulcers and benign gastric ulcers. Pepcid is a competitive inhibitor of histamine H2 receptors that reduces both gastric acid concentration and the volume of gastric secretions.
Q. Complete the Lewis structure of famotidine showing all valence electrons and any formal positive or negative charges.
The structural formulas for ethanol, CH3CH2OH, and propene,
CH;CH=CH,2, are
нн
H
Н—С—С—0—н
H-C-C=C-H
нн
H H H
Ethanol
Propene
(a) Complete the Lewis structure for each molecule showing all valence
electrons.
(b) Using the VSEPR model, predict all bond angles in each molecule.
Chapter 7 Solutions
CHEMISTRY-TEXT
Ch. 7 - Use the electro negativity values in Figure 7.4...Ch. 7 - Conceptual APPLY 7.2 An electrostatic potential...Ch. 7 - The dipole moment of AgCI in the gas phaseis...Ch. 7 - Predict which bond has greater percent ionic...Ch. 7 - Select the correct electron-dot structure for H2S...Ch. 7 - Use the octet rule to predict the molecular...Ch. 7 - Identify the correct electron-dot structure for...Ch. 7 - Draw an electron-dot structure for each of the...Ch. 7 - Select the correct electron-dot structure for...Ch. 7 - Identify the correct electron-dot structure(s) for...
Ch. 7 - Prob. 7.11PCh. 7 - Which oxygen species do you predict to be most...Ch. 7 - Draw an electron-dot structure for the following...Ch. 7 - There are two molecules with the formula C2H6O...Ch. 7 - The following structure is a representation of...Ch. 7 - Draw two possible electron-dot structures for the...Ch. 7 - Called “laughing gas, nitrous oxide (N2O) is...Ch. 7 - Draw as many resonance structures as possible for...Ch. 7 - Prob. 7.19PCh. 7 - Prob. 7.20ACh. 7 - Calculate the formal charge on each atom in the...Ch. 7 - Start with the electron-dot structure for the...Ch. 7 - Calculate formal charges on the C and O atoms in...Ch. 7 - Three resonance structures for anisole (Problem...Ch. 7 - The toxicity of the organophosphate insecticides...Ch. 7 - Prob. 7.26PCh. 7 - Prob. 7.27PCh. 7 - The following structure is a representation of the...Ch. 7 - The electron-dot structure for the nerve a gentsar...Ch. 7 - Draw the new electron-dot structures indicated by...Ch. 7 - The following diagram shows the potential energy...Ch. 7 - The following diagram shows the potential energy...Ch. 7 - Two electrostatic potential maps are shown, one of...Ch. 7 - Prob. 7.34CPCh. 7 - Which of the following drawings is most likely to...Ch. 7 - The following ball-and-stick molecular model is a...Ch. 7 - The following hall-and-stick molecular model is a...Ch. 7 - Sinapaldehyde, a compound present in the toasted...Ch. 7 - Vitamin C (ascorbic acid) has the following...Ch. 7 - Match the following descriptions with the type of...Ch. 7 - Why do two atoms come together to form a covalent...Ch. 7 - Explain the difference in the bond dissociation...Ch. 7 - Explain the difference in the bond dissociation...Ch. 7 - Predict which of the following bonds should be...Ch. 7 - Prob. 7.45SPCh. 7 - What general trends in electro negativity occur in...Ch. 7 - Predict the electro negativity of the undiscovered...Ch. 7 - Order the following elements according to...Ch. 7 - Order the following elements according to...Ch. 7 - Which of the following substances contain bonds...Ch. 7 - Use the electro negativity data in Figure 7.4 to...Ch. 7 - Show the direction of polarity for each of the...Ch. 7 - Show the direction of polarity for each of the...Ch. 7 - Which of the substances...Ch. 7 - Which of the substances...Ch. 7 - Order the following compounds according to the...Ch. 7 - Order the following compounds according to the...Ch. 7 - Prob. 7.58SPCh. 7 - Using only the elements Ca, Cl, and Si, give...Ch. 7 - The dipole moment of BrCl is 0.518 D, and the...Ch. 7 - Prob. 7.61SPCh. 7 - Prob. 7.62SPCh. 7 - Prob. 7.63SPCh. 7 - Why does the octet rule apply primarily to...Ch. 7 - Which of the following substances contains an atom...Ch. 7 - Draw electron-dot structures for the following...Ch. 7 - Draw electron-dot structures for the following...Ch. 7 - Identify the correct electron-dot structure for...Ch. 7 - Draw an electron.dot structure for the hydronium...Ch. 7 - Oxalic acid, H2C2O4 , is a mildly poisonous...Ch. 7 - Draw an electron-dot structure for carbon...Ch. 7 - Prob. 7.72SPCh. 7 - Identify the fourth-row elements, X, that form the...Ch. 7 - Write electron-dot structures for molecules with...Ch. 7 - Write electron-dot structures for molecules with...Ch. 7 - Which compound do you expect to have the stronger...Ch. 7 - Which compound do you expect to have the stronger...Ch. 7 - Draw an electron-dot structure for each of the...Ch. 7 - Prob. 7.79SPCh. 7 - Methylphenidate (C14H19NO2) , marketed as Ritalin,...Ch. 7 - Pregabalin (C8H17NO2) , marketed as Lyric a, is an...Ch. 7 - The following molecular model is that of...Ch. 7 - Ibuprofen C 13 H 18 O 2 marketed under such brand...Ch. 7 - Draw as many resonance structures as you can that...Ch. 7 - Draw as many resonance structures as you can for...Ch. 7 - Which of the following pairs of structures...Ch. 7 - Which of the following pairs of structures...Ch. 7 - Draw as many resonance structures as you can that...Ch. 7 - Draw as many resonance structures as you can that...Ch. 7 - Benzene has the following structural formula. Use...Ch. 7 - Draw three resonance structures for sulfur...Ch. 7 - Some mothballs used when storing clothes are made...Ch. 7 - Four different structures (a), (b), (c), and (d)...Ch. 7 - Draw an electron-dot structure for carbon...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Calculate formal charges for the C and O atoms in...Ch. 7 - Draw two electron-dot resonance structures that...Ch. 7 - Draw two electron-dot resonance structures that...Ch. 7 - Prob. 7.102SPCh. 7 - Prob. 7.103SPCh. 7 - Boron trifluoride reacts with dimethyl ether to...Ch. 7 - Thiofulminic acid, HCNS, has recently been...Ch. 7 - Draw two rcsonancc strutur for methyl isocyanate,...Ch. 7 - In the cyanatc ion. OCN , carbon is the central...Ch. 7 - Prob. 7.108MPCh. 7 - Prob. 7.109MPCh. 7 - Prob. 7.110MPCh. 7 - The neutral OH molecule has been implicated in...Ch. 7 - Prob. 7.112MPCh. 7 - Prob. 7.113MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw Lewis structures for the following species. (The skeleton is indicated by the way the molecule is written.) (a) Cl2CO (b) H3C—CN (c) H2C—CH2arrow_forwardAcrylamide, H2C=CHCONH2, is a known neurotoxin and possible carcinogen. It was a shock to all consumers of potato chips and french fries a few years ago when it was found to occur in those products. (a) Sketch the molecular structure of acrylamide and identify all bond angles. (b) Indicate which carbon-carbon bond is the stronger of the two. (c) Is the molecule polar or nonpolar? (d) The amount of acrylamide found in potato chips is 1.7 mg/kg. If a serving of potato chips is 28 g, how many moles of acrylamide are you consuming?arrow_forwardhat does it mean to say that a bond is polar? Give two examples of molecules with polar bonds. Indicate in your examples the direction of the polarity.arrow_forward
- Pipeline, the active ingredient in black pepper, has this structural formula (a) Write the molecular formula of piperine. (b) Identify the shortest carbon-to-carbon bond in piperine. (c) Identify the shortest carbon-to-oxygen bond in piperine. (d) Identify the strongest carbon-to-carbon bond in piperine. (e) Identify the most polar bond in piperine.arrow_forwardFollowing is a structural formula of benzene, C6H6, which we study in Chapter 21. (a) Using VSEPR, predict each HCC and CCC bond angle in benzene. (b) State the hybridization of each carbon in benzene. (c) Predict the shape of a benzene molecule. (d) Draw important resonance contributing structures.arrow_forward3. The skeleton of chloromethane is __________________ The central carbon atom is bonded to each of the other atoms by a shared electron pair (represented by a straight line, ___) giving Now, each hydrogen has two electrons and the carbon atom has eight. However, chlorine must be provided with unshared electrons (represented by pairs of dots, ) to complete its octet, thusarrow_forward
- Molecules in space: (a) In addition to molecules such as CO, HCl, H2O, and NH3, glycolaldehyde has been detected in outer space. Is the molecule polar? (b) Where do the positive and negative charges lie in the molecule? (c) One molecule found in the 1995 Hale-Bopp comet is HC3N. Suggest a structure for this molecule.arrow_forwardThe study of carbon-containing compounds and their properties is called organic chemistry. Besides carbon atoms, organic compounds also can contain hydrogen, oxygen, and nitrogen atoms (as well as other types of atoms). A common trait of simple organic compounds is to have Lewis structures where all atoms have a formal charge of zero. Consider the following incomplete Lewis structure for an organic compound called histidine (an amino acid), which is one of the building blocks of proteins found in our bodies: Draw a complete Lewis structure for histidine in which all atoms have a formal charge of zero.arrow_forwardExperimental evidence indicates the existence of HC3N molecules in interstellar clouds. Write a plausible Lewis structure for this molecule.arrow_forward
- Oxalic acid, H2C2O4, a poisonous colorless solid, is found in some vegetables such as spinach and rhubarb. It is present in concentrations well below the toxic limit, so you can't use this as a reason to refuse a helping of spinach. The order of atoms in a molecule of oxalic acid is HO2CCO2H. (a) How many unshared pairs of electrons are on each of the carbon atoms? (b) How many unshared pairs of electrons are on each of the oxygen atoms?arrow_forwardiii. Vinyl chloride is the starting material for the production of poly(vinyl chloride), abbreviated PVC. Its recycling code is "V". The major use of PVC is for tubing in residential and commercial construction. H Cl H H Vinyl chloride (a) Complete the Lewis structure for vinyl chloride by showing all unshared pairs of electrons. (b) Predict the H-C-H, H-C-C and Cl-C-H, bond angles in this molecule. (c) Does vinyl chloride have polar bonds? (d) Is it a polar molecule? (e) Does it have a dipole? iv. Diazene (N,H,) and hydrazine (NH,NH,) are reactive nitrogen compounds. Use the hybrid orbitals theory to compare the bonding in these two molecules, and describe the differences in their molecular structures. v. Which molecules in the following figure show an increase in bond order when one electron is added to the molecule? (Hint: Use the molecular orbitals theory.) Liz Bez B3 C2 N2 O2 Nez vi. Use the molecular orbitals theory to decide whether NF would be stabilized or destabilized by…arrow_forwardChrysene is an aromatic hydrocarbon found in coal tar. Convert the molecular model to a Lewis structure in which all of the rings correspond to Kekulé formulas of benzene.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY