
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 86GQ
Molecules in space:
- (a) In addition to molecules such as CO, HCl, H2O, and NH3, glycolaldehyde has been detected in outer space. Is the molecule polar?
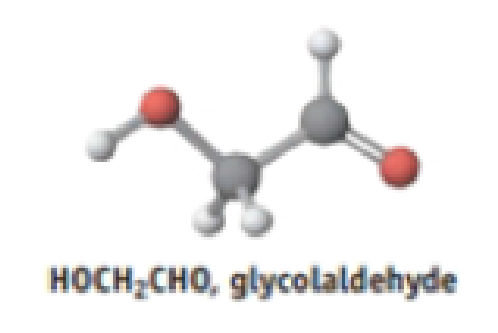
- (b) Where do the positive and negative charges lie in the molecule?
- (c) One molecule found in the 1995 Hale-Bopp comet is HC3N. Suggest a structure for this molecule.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Frenkel and Schottky are intrinsic or extrinsic defects, point or linear defects.
Select the correct option:a) Frenkel and Schottky defects are linear crystal defects.b) Schottky defects involve atomic motions in a crystal lattice.c) Frenkel defects are vacancies in a crystal lattice.d) None of the above is correct.
The most common frequency in organic chemistry is the
Select one:
Oa. carbon-oxygen single bond
Ob. None of the above
Oc.
carbon-carbon double bond
Od. carbon-carbon single bond
Chapter 8 Solutions
Chemistry & Chemical Reactivity
Ch. 8.2 - Draw Lewis electron dot structures for CH3Cl...Ch. 8.2 - Prob. 8.2CYUCh. 8.2 - Prob. 8.3CYUCh. 8.2 - Prob. 8.4CYUCh. 8.3 - Prob. 8.5CYUCh. 8.4 - Draw resonance structures for the bicarbonate ion,...Ch. 8.5 - Sketch the Lewis structures for CIF2+ and CIF2....Ch. 8.6 - What is the shape of the dichloromethane (CH2C12)...Ch. 8.6 - Give the electron-pair geometry and molecular...Ch. 8.6 - Draw the Lewis structure for lCl2, and then decide...
Ch. 8.7 - For each of the following pairs of bonds, decide...Ch. 8.7 - Draw the resonance structures for SCN. What are...Ch. 8.8 - For each of the following molecules, decide...Ch. 8.8 - The electrostatic potential surface for SOCl2 is...Ch. 8.9 - Using the bond dissociation enthalpies in Table...Ch. 8.10 - Prob. 1.1ACPCh. 8.10 - Do any of the atoms in an ibuprofen molecule have...Ch. 8.10 - What is the most polar bond in the molecule?
Ch. 8.10 - Prob. 1.4ACPCh. 8.10 - Prob. 1.5ACPCh. 8.10 - Prob. 1.6ACPCh. 8.10 - Are there any 120° bond angles in ibuprofen? Any...Ch. 8.10 - Prob. 1.8ACPCh. 8.10 - Prob. 2.2ACPCh. 8.10 - Calculate the difference in electronegativity...Ch. 8.10 - Predict the bond dissociation enthalpy for a...Ch. 8.10 - Prob. 3.3ACPCh. 8 - Give the periodic group number and number of...Ch. 8 - Give the periodic group number and number of...Ch. 8 - For elements in Groups 4A-7A of the periodic...Ch. 8 - Prob. 4PSCh. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Show all possible resonance structures for each of...Ch. 8 - Show all possible resonance structures for each of...Ch. 8 - Prob. 11PSCh. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Prob. 18PSCh. 8 - Prob. 19PSCh. 8 - The following molecules or ions all have three...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Give approximate values for the indicated bond...Ch. 8 - Give approximate values for the indicated bond...Ch. 8 - Phenylalanine is one of the natural amino acids...Ch. 8 - Acetylacetone has the structure shown here....Ch. 8 - For each pair of bonds, indicate the more polar...Ch. 8 - For each of the bonds listed below, tell which...Ch. 8 - Urea, (NH2)2CO, is used in plastics and...Ch. 8 - Considering both formal charges and bond...Ch. 8 - Considering both formal charge and bond...Ch. 8 - Three resonance structures are possible for...Ch. 8 - Three resonance structures are possible for the...Ch. 8 - Compare the electron dot structures of the...Ch. 8 - Compare the electron dot structures of the...Ch. 8 - The chemistry of the nitrite ion and HNO2: (a) Two...Ch. 8 - Draw the resonance structures for the formate ion,...Ch. 8 - Prob. 39PSCh. 8 - Consider the following molecules: (a) CH4 (b)...Ch. 8 - Which of the following molecules is(are) polar?...Ch. 8 - Prob. 42PSCh. 8 - Give the bond order for each bond in the following...Ch. 8 - Prob. 44PSCh. 8 - In each pair of bonds, predict which is shorter....Ch. 8 - In each pair of bonds, predict which is shorter....Ch. 8 - Prob. 47PSCh. 8 - Compare the carbon-oxygen bond lengths in the...Ch. 8 - Consider the carbon-oxygen bond in formaldehyde...Ch. 8 - Compare the nitrogen-nitrogen bond in hydrazine,...Ch. 8 - Ethanol can be made by the reaction of ethylene...Ch. 8 - Methanol can be made by partial oxidation of...Ch. 8 - Hydrogenation reactions, which involve the...Ch. 8 - Phosgene, Cl2CO, is a highly toxic gas that was...Ch. 8 - The compound oxygen difluoride is quite reactive,...Ch. 8 - Oxygen atoms can combine with ozone to form...Ch. 8 - Prob. 57GQCh. 8 - Prob. 58GQCh. 8 - Which of the following compounds or ions do not...Ch. 8 - Prob. 60GQCh. 8 - Draw resonance structures for the formate ion,...Ch. 8 - Prob. 62GQCh. 8 - Prob. 63GQCh. 8 - What is the principle of electroneutrality? Use...Ch. 8 - Prob. 65GQCh. 8 - Draw resonance structures for the SO2 molecule,...Ch. 8 - What are the orders of the NO bonds in NO2 and...Ch. 8 - Which has the greater ONO bond angle, NO2 or NO2+?...Ch. 8 - Compare the FClF angles in CIF2+ and ClF2. Using...Ch. 8 - Draw an electron dot structure for the cyanide...Ch. 8 - Draw the electron dot structure for the sulfite...Ch. 8 - Dinitrogen monoxide, N2O, can decompose to...Ch. 8 - The equation for the combustion of gaseous...Ch. 8 - The cyanate ion, OCN, has the least...Ch. 8 - Vanillin is the flavoring agent in vanilla extract...Ch. 8 - Explain why (a) XeF2 has a linear molecular...Ch. 8 - The formula for nitryl chloride is ClNO2 (in which...Ch. 8 - Hydroxyproline is a less-common amino acid. (a)...Ch. 8 - Amides are an important class of organic...Ch. 8 - Prob. 81GQCh. 8 - The molecule shown here. 2-furylmelhanethiol, is...Ch. 8 - Dihydroxyacetone is a component of quick-tanning...Ch. 8 - It is possible to draw three resonance structures...Ch. 8 - Acrolein is used to make plastics. Suppose this...Ch. 8 - Molecules in space: (a) In addition to molecules...Ch. 8 - 1,2-Dichloroethylene can be synthesized by adding...Ch. 8 - The molecule pictured below is epinephrine, a...Ch. 8 - You are doing an experiment in the laboratory and...Ch. 8 - Prob. 90ILCh. 8 - A paper published in the research Journal Science...Ch. 8 - Uracil is one of the bases in RNA, a close...Ch. 8 - Guanine is present in both DNA and RNA. (a) What...Ch. 8 - Prob. 94ILCh. 8 - Prob. 95SCQCh. 8 - Prob. 96SCQCh. 8 - Bromine-containing species play a role in...Ch. 8 - Acrylamide, H2C=CHCONH2, is a known neurotoxin and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardUnshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. CH. H₂ fo H2 H The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c is HC HC HC CH The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c isarrow_forwardDraw curved arrows for the following reaction step. Arrow-pushing Instructions CH3 CH3 H H-O-H +/ H3C-C+ H3C-C-0: CH3 CH3 Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY