
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 47P
Starting with an appropriate
(a)
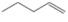
(b)
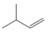
(c)
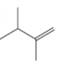
(d)
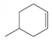
(e)
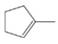
(f)
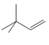
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Show mechanism..don't give Ai generated solution
Don't used Ai solution
Show work. Don't give Ai generated solution
Chapter 7 Solutions
Organic Chemistry
Ch. 7 - Prob. 1PPCh. 7 - Prob. 2PPCh. 7 - Prob. 3PPCh. 7 - Prob. 4PPCh. 7 - Practice Problem 7.5
How many stereoisomers are...Ch. 7 - Prob. 6PPCh. 7 - Prob. 7PPCh. 7 - PRACTICE PROBLEM 7.8
Examine Solved Problem 7.3....Ch. 7 - Prob. 9PPCh. 7 - Practice Problem 7.10 When...
Ch. 7 - Practice Problem 7.11
(a) When...Ch. 7 - Prob. 12PPCh. 7 - Prob. 13PPCh. 7 - Practice Problem 7.14
Dehydration of 2-propanol...Ch. 7 - Practice Problem 7.15
Rank the following alcohols...Ch. 7 - Practice Problem 7.16
Acid-catalyzed dehydration...Ch. 7 - Practice Problem 7.17 Acid-catalyzed dehydration...Ch. 7 - Prob. 18PPCh. 7 - Prob. 19PPCh. 7 - Practice Problem 7.20
Show how you might...Ch. 7 - Prob. 21PPCh. 7 - Prob. 22PPCh. 7 - Practice Problem 7.23
Write the structure of...Ch. 7 - Prob. 24PPCh. 7 - Prob. 25PPCh. 7 - Practice Problem 7.26 (a) Devise retrosynthetic...Ch. 7 - Each of the following names is incorrect, Give the...Ch. 7 - Prob. 28PCh. 7 - Prob. 29PCh. 7 - Give the IUPAC names for each of the following:...Ch. 7 - Prob. 31PCh. 7 - Prob. 32PCh. 7 - Prob. 33PCh. 7 - Prob. 34PCh. 7 - 7.35. Write structural formulas for all the...Ch. 7 - 7.36. Explain the following observations: When...Ch. 7 - Prob. 37PCh. 7 - Arrange the following alcohols in order of their...Ch. 7 - Prob. 39PPCh. 7 - Prob. 40PPCh. 7 - Prob. 41PPCh. 7 - Prob. 42PPCh. 7 - Your task is to prepare isopropyl methyl ether by...Ch. 7 - Prob. 44PCh. 7 - Prob. 45PCh. 7 - Prob. 46PCh. 7 - 7.47. Starting with an appropriate alkyl halide...Ch. 7 - Prob. 48PCh. 7 - 7.49. What is the index of hydrogen deficiency...Ch. 7 - Prob. 50PCh. 7 - Prob. 51PCh. 7 - Compounds I and J both have the molecular formula...Ch. 7 - Prob. 53PCh. 7 - 7.54. Outline a synthesis of phenylethyne from...Ch. 7 - Prob. 55PPCh. 7 - Prob. 56PPCh. 7 - Prob. 57PPCh. 7 - cis-4-Bromocyclohexanol tBuOHtBuO racemic C6H10O...Ch. 7 - Prob. 59PPCh. 7 - Consider the interconversion of cis-2-butene and...Ch. 7 - Prob. 61PCh. 7 - (a) Using reactions studied in this chapter, show...Ch. 7 - Prob. 63PCh. 7 - Prob. 64PCh. 7 - 1. Write the structure(s) of the major product(s)...Ch. 7 - Prob. 2LGPCh. 7 - (a) Write the structure of the product(s) formed...Ch. 7 - Prob. 4LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Q7. What is the mass of a 1.75 L sample of a liquid that has a density of 0.921 g/mL?
a) 1.61 × 103 g
b) 1....
Chemistry: A Molecular Approach (4th Edition)
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
Using the pKa values listed in Table 15.1, predict the products of the following reactions:
Organic Chemistry (8th Edition)
26. What are the strength and direction of an electric field that will balance the weight of (a) a proton and (...
College Physics: A Strategic Approach (3rd Edition)
41. A reaction in which A, B, and C react to form products is first order in A, second order in B, and zero ord...
Chemistry: Structure and Properties (2nd Edition)
10.71 Identify each of the following as an acid or a base: (10.1)
H2SO4
RbOH
Ca(OH)2
HI
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- this is an organic chemistry question please answer accordindly!! please post the solution draw the figures on a paper please hand drawn and post, please answer EACH part till the end and dont just provide wordy explanations, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardA mixture of 0.412 M C12, 0.544 M F2, and 0.843 M CIF is enclosed in a vessel and heated to 2500 K. C12(g) + F2(g )2CIF(g) Kc = 20.0 at 2500 K Calculate the equilibrium concentration of each gas at 2500 K. [C12] = M [F2] = M [ CIF] =arrow_forwardShow reaction mechanism with explanation. don't give Ai generated solutionarrow_forward
- Don't used Ai solutionarrow_forwardthis is an organic chemistry question please answer accordindly!! please post the solution draw the figures and post, answer the question in a very simple and straight forward manner thanks!!!!! please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures or diagrams, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this kindly solve all parts and draw it not just word explanations!!arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY