
Concept explainers
How do positive ions and negative ions form?
Interpretation:
The formation of positive and negative ions needs to be explained.
Concept introduction:
Most of the atoms does not have eight electron in its valence shells to have a stable configuration. For instance, some may have less than 8 in its outer shell which indicates that it will be deficient of 4 electrons or just 1 to 2 electrons. In case the atom needs less than 3 valence electrons, it will tend to lose those electrons to become electrically neutral and the lower shell electrons will form an octet. Or in some cases, it will gain those 2 electrons and become octet.
Answer to Problem 46A
Atoms will lose electrons to become a positive charged ion and it can gain electrons to become a negatively charged ion so that it attains an octet or stable configuration.
Explanation of Solution
As atoms of elements do not have eight electrons in it valence shells, these atoms will tend to lose or gain electrons to form a stable configuration.
Let us consider Sodium atom that has electronic configuration as
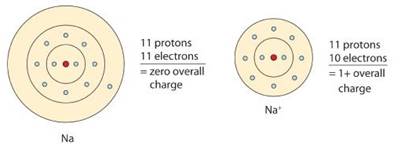
By losing an electron, Sodium atom becomes Sodium ion having an octet structure.
Let us consider Chlorine atom that has electronic configuration as
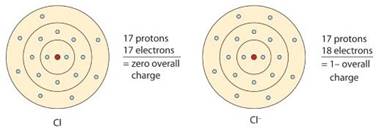
Atoms will lose electrons to become a positive charged ion and it can gain electrons to become a negatively charged ion so that it attains an octet or stable configuration.
Want to see more full solutions like this?
Chapter 7 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
Organic Chemistry
Chemistry: The Central Science (14th Edition)
Inorganic Chemistry
Organic Chemistry (9th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





