
(a)
Interpretation:
The
Concept Introduction:
Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
(a)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for
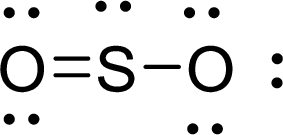
The electron-region geometry of Sulphur atom bonded to two other atoms and one lone pair of electron is triangular planar. It is a type of
(b)
Interpretation:
The
Concept Introduction:
Refer to (a).
(b)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for
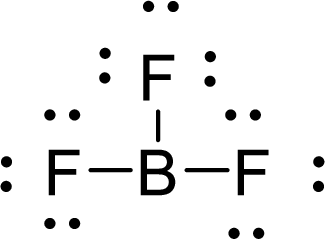 .
.
The electron-region geometry of Boron atom bonded to three other atoms is triangular planar. It is a type of
(c)
Interpretation:
The bond angles of
Concept Introduction:
Refer to (a).
(c)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for
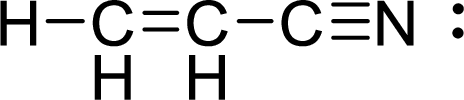 .
.
The electron-region geometry of first Carbon atom bonded to three other atoms is triangular planar. It is a type of
The electron-region geometry of third Carbon atom bonded to two other atoms is linear. It is a type of
(d)
Interpretation:
The bond angles
Concept Introduction:
Refer to (a).
(d)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for
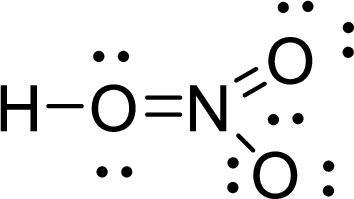 .
.
The electron-region geometry of first Oxygen atom bonded to two other atoms and two lone pair of electrons is tetrahedral. It is a type of
The electron-region geometry of nitrogen atom bonded to three other atoms is triangular planar. It is a type of
Want to see more full solutions like this?
Chapter 7 Solutions
Chemistry: The Molecular Science
- Using line angle formulas, draw thestructures of and name four alkanes that have total of 7carbons, one of which is tertiary.Please explain this in detail and can you also explain how to approach a similar problem like this as well?arrow_forwardUsing dashed line wedge projections drawthe indicated compounds and indicate whether thecompound you have drawn is R or S.(a) The two enantiomers of 2-chlorobutane. Can you please explain your steps and how you would approach a similar problem. Thank you!arrow_forward5) There are no lone pairs shown in the structure below. Please add in all lone pairs and then give the hybridization scheme for the compound. (8) 10,11 7) 1.2.3 H 4 | 14 8) COC 12 13 H 16 15 H7 9) - 5.6 C 8 H 10) H 1). 2) 3)_ 11) 12) 13) 4)_ 14) 5) 15) 16) 6)arrow_forward
- The sum of the numbers in the name of isA. 11; B. 13; C. 10; D. 12; E. none of the other answers iscorrect. I believe the awnser should be E to this problem but the solution to this problem is D 12. I'm honestly unsure how that's the solution. If you can please explain the steps to this type of problem and how to approach a problem like this it would be greatly appreciated!arrow_forwardConsider the following data for phosphorus: g atomic mass 30.974 mol electronegativity 2.19 kJ electron affinity 72. mol kJ ionization energy 1011.8 mol kJ heat of fusion 0.64 mol You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb or release energy? 2+ + (1) P (g) + e → P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (1) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (1): Does the following reaction absorb or release energy? 00 release absorb Can't be decided with the data given. yes no ☐ kJ/mol (²) P* (8) + + + e →>> P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (2) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (2): ☐ release absorb Can't be decided with the data given. yes no kJ/mol аarrow_forwardThe number of hydrogens in an alkyne that has a main chain of 14carbons to which are attached a cyclobutyl ring, a benzene ring, an–OH group, and a Br is A. 34; B. 35; C. 36; D. 24; E. 43arrow_forward
- Hello! I have a 500 Hz H-NMR for 1,5-bis-(4-methoxyphenyl)-penta-1,4-dien-3-one. I need to label the signals with the corresponding H's. Then, find out if the two alkenes are cis or trans by calculating the J values. I believe that I have the H-NMR labeled correctly, but not sure if I got the J values correct to determine if the two alkenes in the compound will make the compound cis or trans.arrow_forwardWhat is the only possible H-Sb-H bond angle in SbH3?arrow_forwardpls helparrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





