
Concept explainers
(a)
Interpretation:The empirical formula of the given molecular model needs to be determined.
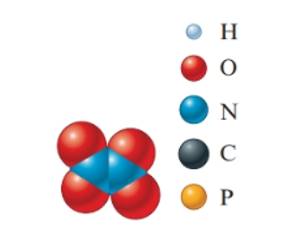
Concept Introduction: The molecular mass of compound is the sum of
Whereas empirical formula can be defined as the simplest positive integer ratio of all bonded atoms in the compound.
(a)
Answer to Problem 56A
The empirical formula must be
Explanation of Solution
In the given molecular model, the molecular formula must be

(b)
Interpretation:The empirical formula of the given molecular model needs to be determined.
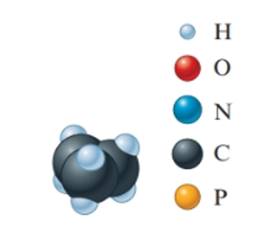
Concept Introduction: The molecular mass of compound is the sum of atomic mass of all the atoms present in the given chemical compound. Here the molecular formula represents the exact number of all bonded atoms in the compound.
Whereas empirical formula can be defined as the simplest positive integer ratio of all bonded atoms in the compound.
(b)
Answer to Problem 56A
The empirical formula must be
Explanation of Solution
In the given molecular model, the molecular formula must be
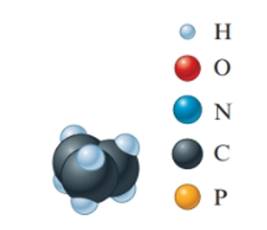
(c)
Interpretation:The empirical formula of the given molecular model needs to be determined.
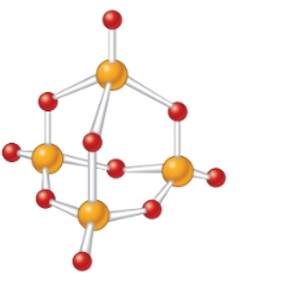

Concept Introduction: The molecular mass of compound is the sum of atomic mass of all the atoms present in the given chemical compound. Here the molecular formula represents the exact number of all bonded atoms in the compound.
Whereas empirical formula can be defined as the simplest positive integer ratio of all bonded atoms in the compound.
(c)
Answer to Problem 56A
The empirical formula must be
Explanation of Solution
In the given molecular model, the molecular formula must be
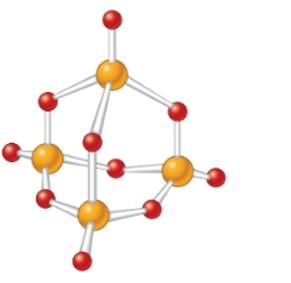
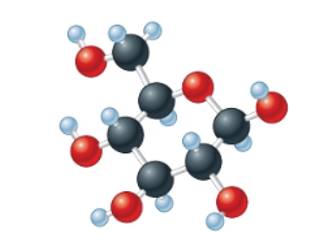
(d)
Interpretation:The empirical formula of the below molecular model needs to be determined.
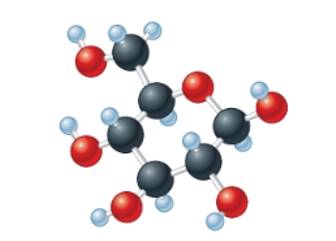

Concept Introduction: The molecular mass of compound is the sum of atomic mass of all the atoms present in the given chemical compound. Here the molecular formula represents the exact number of all bonded atoms in the compound.
Whereas empirical formula can be defined as the simplest positive integer ratio of all bonded atoms in the compound.
(d)
Answer to Problem 56A
The empirical formula must be
Explanation of Solution
In the given molecular model, the molecular formula must be
Chapter 6 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





