
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 2P
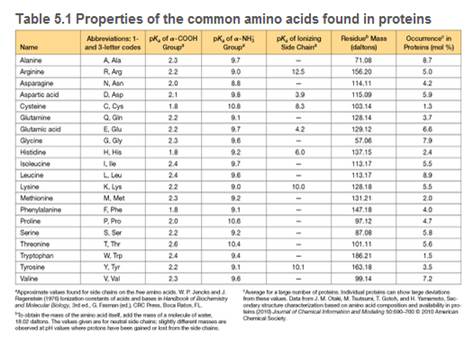
Draw the structure of the peptide DTLH, showing the backbone and side-chain atoms in the ionization states favored at pH = 7.0.
a. Draw a water molecule making a hydrogen bond to a side-chain H-bond donor.
b. Draw a water molecule making a hydrogen bond to a main-chain H-bond acceptor.
c. Using the values of pKas given in Table 5.1, calculate the pl for DTLH.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the following pentapeptides: Ser-Glu-Gly-His-Ala and Gly-His-Ala-Glu-Ser
A. Compute their isoelectric pH (pI). Show full solution. Use standard pKa values.
B. Do these peptides with the same amino acid composition have different net charges at pH 7.0? Explain briefly.
C. Would you expect the titration curves of the two peptides to differ? Why or Why not?
Identify and describe how you would PEGylate this peptide at its N- terminal amine. Discuss the reaction conditions you need to carry out the reaction and explain how pH affect selectivity of reaction. Draw the chemical structure of the resulting mPEG peptide conjugate.
Peptides have been discovered that display anti-inflammatory properties and show promise as new drugs. One such peptide is the 5-mer (pentapeptide): MTADV. a. Draw the structure of MTADV in its predominant form at pH 10. b. What is the net charge on this peptide at pH 10?
Chapter 5 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 5 - Prob. 1PCh. 5 - Draw the structure of the peptide DTLH, showing...Ch. 5 - Prob. 3PCh. 5 - Prob. 4PCh. 5 - Prob. 5PCh. 5 - Prob. 6PCh. 5 - Prob. 7PCh. 5 - Given the following peptide SEPIMAPVEYPK a....Ch. 5 - A mutant form of polypeptide hormone angiotensin...Ch. 5 - Prob. 10P
Ch. 5 - Prob. 11PCh. 5 - a. Write a possible sequence for an mRNA segment...Ch. 5 - 13. Assume the following portion of an mRNA Find a...Ch. 5 - Prob. 14PCh. 5 - Prob. 15PCh. 5 - Prob. 16PCh. 5 - Prob. 17PCh. 5 - Prob. 18PCh. 5 - You are a summer intern in a clinical hematology...Ch. 5 - Prob. 20PCh. 5 - Despite the fact that many peptides have critical...Ch. 5 - Based on the information in Figure 5.17, which...Ch. 5 - If you want to purify a DNA-binding protein from a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- For the peptide Ala-Cys-His-Ile-Leu-Asp a. Write the single letter code for the amino acid residues b. What is the charge of the peptide at pH 7.0. Assign the following pKa values: 3,4,6,8,9 c. What is the pI of the peptidearrow_forwardA. Write the structure of the following peptide at pH 5.0 and calculate its net charge at this pH. Asp-His-Tyr-Arg-Lys-Leu-Thr-Gln. Based on the pKa value of the ionizable groups. B. A polypeptide consisting only of L-glutamate residues (poly-L-glutamate) may have a random coil or helical structure depending on pH. Explain this behavior by indicating at what pH values the helical structure will be favored.arrow_forwardDraw the structure of the peptide DTLH, showing the backbone and sidechain atoms in the ionization states favored at pH = 7.0. (a) Draw a water molecule making a hydrogen bond to a side-chain H-bond donor. (b) Draw a water molecule making a hydrogen bond to a main-chain H-bond acceptor. (c) Using the values of pKas given in Table, calculate the pI for DTLHarrow_forward
- Draw the structure of the peptide DTLH, showing the backbone and sidechainatoms in the ionization states favored at pH = 7.0.(a) Draw a water molecule making a hydrogen bond to a side-chain H-bonddonor.(b) Draw a water molecule making a hydrogen bond to a main-chain H-bond acceptor. (c) Using the values of pKas given in as shown, calculate the pI for DTLH.arrow_forwardAt which pH would the peptide with the amino acid sequence 'KEYHR' be found in an IPG (Immobilized pH gradient) strip after successful isoelectric focusing. Show calculation. lonizable groups Alpha amine group of K Side chain group of K Side chain group of E Side chain group of Y Side chain group of H Side chain group of R Alpha carboxylic acid group of R pka 8.95 10.53 4.25 10.07 6 12.48 2.17arrow_forwardA peptide with an amino acid sequence and molecular mass (1028.5193 Da) is given below HVMTLNLGEΚ Determine the maximum number of positive charges of this peptide. а. b. Determine the possible m/z ratio of this peptide at different charge states.arrow_forward
- You have a peptide that has the following amino acid sequence: GPMG Draw the structure of the polypeptide's most protonated form, and its charge. Then draw the structures of the increasingly deprotonated forms, along with their charges. Use the information from these structures to calculate the pl (isoelectric point) of the peptide using the pKa values provided in the table below. Write the pl as x.yz, for example, 7.62 or 3.09. Group Terminal a carboxyl group Aspartic acid Glutamic acid Histidine Terminal a-amino group Cysteine Lysine Tyrosine Arginine Acid EM 2-0" + H -N H H N-H H N-H T Base 1 1. تر H -5 H H O™ N-H Typical pK, 3.1 4.1 6.0 8.0 8.3 10.8 10.9 12.5arrow_forwardPlease calculate and plot the charge on a peptide with the sequence of NEYK over the pH range from 1 to 13. Please use the pKa values from the table. You may calculate the charge at 1 pH unit intervals.arrow_forwardGiven the following Peptide: Q6 *H;N-Phe-Asp-Ala-Arg-Gy-His-Arg-Asp-Glu-His-Tyr-CO 6a. What is the peptide's net charge at pH 2.0, pH 6.0, pH 7.4, and pH 10.2? (show work) 6b. What is the approximate isoelectric point of this peptide?arrow_forward
- A protein has molecular mass of 200 kDa when measured by gel filtration. When subjected to SDS PAGE with and without 2-mercaptoethanol (2-ME) the gel shown below was obtained. What is the likely subunit composition of this protein and why? a. The protein has 4 subunits, with molecular masses 100, 50, 25, and 25 kDa. 25 kDa subunits are linked to each other via noncovalent interactions. b. The protein has 4 subunits, with molecular masses 100, 50, 25, and 25 kDa. 50 kDa subunit is linked to the one 25 kDa subunit via noncovalent interactions. c. The protein has 3 subunits, with molecular masses 100, 75 and 25 kDa that are linked by noncovalent interactions. d. The protein has 4 subunits, with molecular masses 100, 75, 50 and 25 kDa. The subunits are linked by disulfide bonds. e. The protein has 4 subunits, with molecular masses 100, 50, 25, and 25 kDa. 50 kDa subunit is linked to the one 25 kDa subunit by disulfide bonds.arrow_forwardDetermine the net charge (to the nearest integer) on the following peptide at pH 5 AND pH 12. Estimate the isoelectric point (pl) for this peptide: H2N-Leu-Gly-Lys-Glu-COOH Assume the pK,s for functional groups are: alpha-amino 6. alpha-carboxy sidechain-amino (Lys) 10.5 sidechain-carboxy (Glu) 4.2 O Net charge at pH 5 and 12 is 0 and -2, respectively, pl =6.6 O Net charge at pH 5 and 12 is 0 and -2, respectively, pl =3.1 O Net charge at plH 5 and 12 is +2 and -1, respectively, pl =7.5 O Nct charge at pH 5 and 12 is +1 and -1. respectively,. pl -4.5 2.arrow_forwardDiscuss the reaction conditions you would need to carry out the PEGylation reaction of this peptide and explain how the pH affects the selectivity of these reactions.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY