
(a) Interpretation:
The amino acid in Fischer projection and interpret it as D- or L- enantiomer.
Concept introduction:
Fischer projections are the representation of bonds by using horizontal and vertical lines in the 3D state. The horizontal lines represent the bonds that are out of paper towards the vision and the vertical lines represent the bonds pointing out the back of the paper away from vision. The intersection between the two lines represents the carbon atom.
A carbon which has all the four different atoms or group of atoms has tetrahedral geometry and such carbon molecule is referred to as the chiral carbon. The two different forms in which a single chiral center can exist is referred to as enantiomers. Simply, the substances which show optical isomerism exist as two isomers known as enantiomers. Enantiomers are non-super imposable mirror images of each other. The number of enantiomers of a molecule depends on the number of chiral centers.
Pictorial representation:
The Fischer projection of the given amino acidvaline is represented as below:
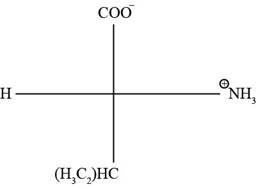
Fig. 1(a): Fischer projection of valine.
(b) Interpretation:
The amino acid in Fischer projection and interpret it as D- or L- enantiomer.
Concept introduction:
Fischer projections are the representation of bonds by using horizontal and vertical lines in the 3D state. The horizontal lines represent the bonds that are out of paper towards the vision and the vertical lines represent the bonds pointing out the back of the paper away from vision. The intersection between the two lines represents the carbon atom.
A carbon which has all the four different atoms or group of atoms has tetrahedral geometry and such carbon molecule is referred to as the chiral carbon. The two different forms in which a single chiral center can exist is referred to as enantiomers. Simply, the substances which show optical isomerism exist as two isomers known as enantiomers. Enantiomers are non-super imposable mirror images of each other. The number of enantiomers of a molecule depends on the number of chiral centers.
Pictorial representation:
The Fischer projection of threonine is represented as below.
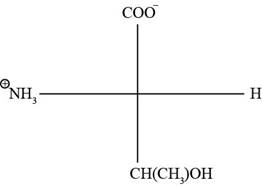
Fig. 2(a): Fischer projection of threonine.
(c) Interpretation:
The amino acid in Fischer projection and interpret it as D- or L- enantiomer.
Concept introduction:
Fischer projections are the representation of bonds by using horizontal and vertical lines in the 3D state. The horizontal lines represent the bonds that are out of paper towards the vision and the vertical lines represent the bonds pointing out the back of the paper away from vision. The intersection between the two lines represents the carbon atom.
A carbon which has all the four different atoms or group of atoms has tetrahedral geometry and such carbon molecule is referred to as the chiral carbon. The two different forms in which a single chiral center can exist is referred to as enantiomers. Simply, the substances which show optical isomerism exist as two isomers known as enantiomers. Enantiomers are non-super imposable mirror images of each other. The number of enantiomers of a molecule depends on the number of chiral centers.
Pictorial representation:
The Fischer projection of glutamine is represented as below:
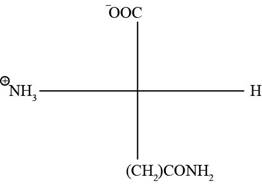
Fig. 3(a): Fischer projection of glutamine.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





