
Chemical Principles
8th Edition
ISBN: 9781305581982
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5, Problem 129AE
Interpretation Introduction
Interpretation: The plot that does not represents correctly Boyle’s law needs to be determined from the following:
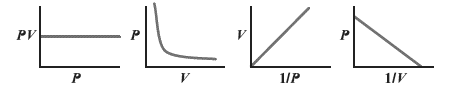
Concept Introduction: The law which states the relation between pressure,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Chemical Principles
Ch. 5 - Consider the following apparatus: a test tube...Ch. 5 - Prob. 2DQCh. 5 - Prob. 3DQCh. 5 - Prob. 4DQCh. 5 - Prob. 5DQCh. 5 - Prob. 6DQCh. 5 - Prob. 7DQCh. 5 - Prob. 8DQCh. 5 - Prob. 9DQCh. 5 - Prob. 10DQ
Ch. 5 - Prob. 11DQCh. 5 - Prob. 12DQCh. 5 - Prob. 13DQCh. 5 - Prob. 14DQCh. 5 - Prob. 15DQCh. 5 - Prob. 16DQCh. 5 - Prob. 17DQCh. 5 - For each of the quantities (af) listed below,...Ch. 5 - Prob. 19DQCh. 5 - Prob. 20DQCh. 5 - A sealed-tube manometer as shown below can be...Ch. 5 - A diagram for an open-tube manometer is shown...Ch. 5 - Prob. 23ECh. 5 - Prob. 24ECh. 5 - A gauge on a compressed gas cylinder reads 2200...Ch. 5 - Prob. 26ECh. 5 - Prob. 27ECh. 5 - Prob. 28ECh. 5 - Prob. 29ECh. 5 - Prob. 30ECh. 5 - A mixture of 1.00 g H2 and 1.00 g He is placed in...Ch. 5 - Prob. 32ECh. 5 - Prob. 33ECh. 5 - Prob. 34ECh. 5 - A piece of solid carbon dioxide, with a mass of...Ch. 5 - Prob. 36ECh. 5 - Suppose two 200.0-L tanks are to be filled...Ch. 5 - Prob. 38ECh. 5 - Prob. 39ECh. 5 - Prob. 40ECh. 5 - Prob. 41ECh. 5 - Prob. 42ECh. 5 - Prob. 43ECh. 5 - Prob. 44ECh. 5 - Prob. 45ECh. 5 - A sample of nitrogen gas was collected over water...Ch. 5 - Prob. 47ECh. 5 - Prob. 48ECh. 5 - Prob. 49ECh. 5 - A 1.00-L gas sample at 100.°C and 600. torr...Ch. 5 - Prob. 51ECh. 5 - Given that a sample of air is made up of nitrogen,...Ch. 5 - Prob. 53ECh. 5 - Prob. 54ECh. 5 - A compound contains only nitrogen and hydrogen and...Ch. 5 - A compound has the empirical formula CHCl. A...Ch. 5 - One of the chemical controversies of the...Ch. 5 - Discrepancies in the experimental values of the...Ch. 5 - A sample of methane (CH4) gas contains a small...Ch. 5 - Prob. 60ECh. 5 - Prob. 61ECh. 5 - Urea (H2NCONH2) is used extensively as a...Ch. 5 - Methanol (CH3OH) can be produced by the...Ch. 5 - Consider the reaction between 50.0 mL of liquid...Ch. 5 - Some very effective rocket fuels are composed of...Ch. 5 - Air bags are activated when a severe impact causes...Ch. 5 - Prob. 67ECh. 5 - Prob. 68ECh. 5 - Prob. 69ECh. 5 - Xenon and fluorine will react to form binary...Ch. 5 - The nitrogen content of organic compounds can be...Ch. 5 - Prob. 72ECh. 5 - Prob. 73ECh. 5 - Consider the following balanced equation in which...Ch. 5 - Prob. 75ECh. 5 - Prob. 76ECh. 5 - Prob. 77ECh. 5 - Prob. 78ECh. 5 - Prob. 79ECh. 5 - Prob. 80ECh. 5 - Calculate the average kinetic energies of the...Ch. 5 - Prob. 82ECh. 5 - Prob. 83ECh. 5 - Prob. 84ECh. 5 - Prob. 85ECh. 5 - Prob. 86ECh. 5 - Prob. 87ECh. 5 - One way of separating oxygen isotopes is by...Ch. 5 - A compound contains only C, H, and N. It is 58.51%...Ch. 5 - Prob. 90ECh. 5 - Prob. 91ECh. 5 - Prob. 92ECh. 5 - Why do real gases not always behave ideally?...Ch. 5 - Prob. 94ECh. 5 - Prob. 95ECh. 5 - Without looking at tables of values, which of the...Ch. 5 - Prob. 97ECh. 5 - Prob. 98ECh. 5 - Prob. 99ECh. 5 - Prob. 100ECh. 5 - Prob. 101ECh. 5 - Prob. 102ECh. 5 - Consider separate 1.0-L samples of O2(g) and...Ch. 5 - Consider separate 1.00-L samples of Ar(g), both...Ch. 5 - Calculate the intermolecular collision frequency...Ch. 5 - Prob. 106ECh. 5 - Prob. 107ECh. 5 - Prob. 108ECh. 5 - Prob. 109ECh. 5 - Prob. 110ECh. 5 - Prob. 111ECh. 5 - Prob. 112AECh. 5 - Prob. 113AECh. 5 - Prob. 114AECh. 5 - Prob. 115AECh. 5 - Prob. 116AECh. 5 - Prob. 117AECh. 5 - Prob. 118AECh. 5 - A 2.747-g sample of manganese metal is reacted...Ch. 5 - Prob. 120AECh. 5 - At STP, 1.0 L Br2 reacts completely with 3.0 L F2...Ch. 5 - Prob. 122AECh. 5 - Prob. 123AECh. 5 - Prob. 124AECh. 5 - Prob. 125AECh. 5 - Prob. 126AECh. 5 - Prob. 127AECh. 5 - Prob. 128AECh. 5 - Prob. 129AECh. 5 - Prob. 130AECh. 5 - Prob. 131AECh. 5 - Prob. 132AECh. 5 - Prob. 133AECh. 5 - Prob. 134AECh. 5 - Prob. 135AECh. 5 - Prob. 136AECh. 5 - Prob. 137AECh. 5 - Prob. 138AECh. 5 - Prob. 139AECh. 5 - Prob. 140AECh. 5 - Prob. 141AECh. 5 - Prob. 142AECh. 5 - Prob. 143AECh. 5 - Prob. 144AECh. 5 - Prob. 145AECh. 5 - Prob. 146CPCh. 5 - A 16.0-g sample of methane (CH4) reacts with 64.0...Ch. 5 - You have two samples of helium gas at the same...Ch. 5 - Prob. 149CPCh. 5 - Prob. 150CPCh. 5 - Prob. 151CPCh. 5 - Prob. 152CPCh. 5 - The density of a pure gaseous compound was...Ch. 5 - Prob. 154CPCh. 5 - The most probable velocity ump is the velocity...Ch. 5 - Derive Dalton’s law of partial pressures from the...Ch. 5 - One of the assumptions of the kinetic molecular...Ch. 5 - Prob. 158CPCh. 5 - A steel cylinder contains 5.00 moles of graphite...Ch. 5 - Prob. 160CPCh. 5 - Prob. 161CPCh. 5 - Prob. 162CPCh. 5 - Calculate the number of stages needed to change...Ch. 5 - Prob. 164CPCh. 5 - You have a helium balloon at 1.00 atm and 25°C....Ch. 5 - Prob. 166CPCh. 5 - Prob. 167MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- perform stoichiometric ca1cu1uions for reactions involving gases as reactants or products.arrow_forwardUnder what conditions does the behavior of a real gas begin to differ significantly from the ideal gas law?arrow_forwardExplain why the plot of PV for CO2 differs from that of an ideal gas.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co