
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 6P
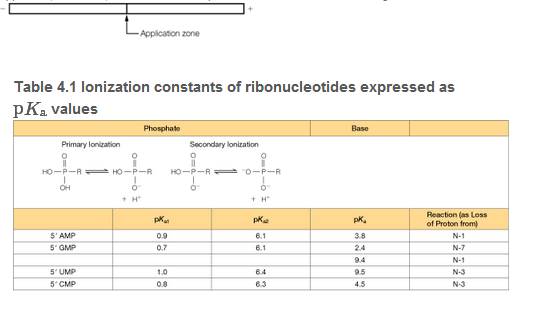
Using the pKa data in Table 4.1 and the Henderson-Hasselbalch equation, calculate the approximate net charge on each of the four common ribonucleoside 5'-monophosphates (rNMPs) at pH 3.8. If a mixture of these rNMPs was placed in an electrophoresis apparatus, as shown, draw four bands to predict the direction and relative migration rate of each.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Bovine ribonuclease folds with ΔH° = -280 kJ mol-1 and ΔS° = -0.79 kJ mol-1 K -1. Assume ΔH° and ΔS° are independent of temperature. What fraction of bovine ribonuclease is unfolded at 42 °C?
A ribonuclease reaction containing 0.50µM RNASE A will hydrolyze RNA at a maximal rate of 5µM/s in the presence of 40mM RNA at 37°C. What is
the turnover number, kcat, for the enzyme (per second).
Electrophoresis is performed at PH 6.8 on a mixture of mutated hemoglobin that differ from normal haemoglobin (Hb) only by the substitution of one amino acid- Hb X: Val replaced par Glu
- Hb Y: Asp replaced by Leu
- Hb Z: Glu replaced by Lys
What will be the order of migration between cathode and anode of these mutated Hb compared to normal Hb? Justify your answer.
Chapter 4 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 4 - Prob. 1PCh. 4 - What is the difference between a nucleoside...Ch. 4 - pppApCpCpupApGpApu-OH a. Using the straight-chain...Ch. 4 - Shown is a representation of a molecule being...Ch. 4 - Base analysis of DNA from maize (com) shows it to...Ch. 4 - Using the pKa data in Table 4.1 and the...Ch. 4 - For some DNAs, it is possible to separate the two...Ch. 4 - Refer to Figure 4.15, which presents the...Ch. 4 - Suppose that you centrifuged a transfer RNA...Ch. 4 - Predict the structure of a cruciform that could be...
Ch. 4 - DNA from a newly discovered virus was purified,...Ch. 4 - Would you expect Neurospora crassa DNA to have a...Ch. 4 - A circular double-stranded DNA molecule contains...Ch. 4 - The gel electrophoresis pattern in Figure 4.23 was...Ch. 4 - 15. DNA polymerase requires both a template, to be...Ch. 4 - Prob. 16PCh. 4 - Prob. 17PCh. 4 - a. What two enthalpic factors stabilize DNA in...Ch. 4 - 19.
a. The plasmid pBR322 (4362 base pairs) was...Ch. 4 - Prob. 20PCh. 4 - What DNA sequence feature is required for a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the pelleting time for eukaryotic ribosome subunits, 40S and 60S, in a rotor with k = 180? Assume that the medium viscosity and density are similar to water at %3D 20°C.arrow_forwardTo approximate the concentration of enzymes in a bacterial cell, assume that the cell contains equal concentrations of 1,000 different enzymes in solution in the cytosol and that each protein has a molecular weight of 100,000. Assume also that the bacterial cell is a cylinder (diameter 1.0 μm, height 2.0 μm), that the cytosol (specific gravity 1.20) is 20% soluble protein by weight, and that the soluble protein consists entirely of enzymes. Calculate the average molar concentration of each enzyme in this hypothetical cell.arrow_forwardPfCRT is found to have 10 peaks on a hydropathy plot given its primary sequence. Researchers also noted that each peak is comprised of about 30 amino acids. The digestive vacuole membrane is 45Å in Plasmodium species. Explain why researchers conclude there are 10 transmembrane helices from these two pieces of data.arrow_forward
- The ESI-MS spectrum in positive ionization mode for lysozyme is obtained. a. What is the molecular weight of the protein to 5 significant figures based on the two highlighted ion species? b. What is the charge of the peaks at 1101.5 and 1789.2.arrow_forwardWhat would the order of migration be (bottom to top in the gel) in a SDS-PAGE for the following proteins? Concanavalin A (ConA) (a homotetramer of 106 kDa), lysozyme (a monomer of 14.3 kDa) & horse liver alcohol dehydrogenase (ADH) (a homodimer of 80 kDa). a. ADH, ConA, lysozyme b. ConA, ADH, lysozyme c. Lysozyme, ADH, ConA Od. Lysozyme, ConA, ADHarrow_forwardAn Fab fragment binds to lysozyme with a dissociation constant of Ka = 10-11 M. A 1 nM (10-9 M) solution of lysozyme is treated with increasing concentrations of the Fab fragment. At what concentration of added Fab will half of the lysozyme be bound to the Fab? [Fab] 9.9 Incorrect nMarrow_forward
- Purification of a protein of unknown structure has been achieved. The natural protein has a molecular weight of 240,000, according to size-exclusion chromatography. Using a concentration of 6 M guanidine hydrochloride in the chromatography, a single peak can be identified as the molecular weight (MW) 60,000 of a protein. B-mercaptoethanol (BME) and guanidine hydrochloride (GHC) are used in tandem to produce proteins with mass masses of 34,000 and 26,000, respectively. The structure of this protein can be inferred from these facts.arrow_forwardThe diffusivity of amino acids in polyacrylamide gel is approximately 1x10^-9 cm2./s calculate the initial flux of amino acids, give an instantaneous gradient of (20g/cm 3 )/8cm Why is polyacrylamide gel is used in electrophoresis?arrow_forwardDraw graphs of optical densities of the amino acid ninhydrin complexes at different wavelengthsarrow_forward
- Which of the following statements regarding Anfinsen's denaturing experiments with ribonuclease A are valid? (i) Exposing the denatured protein to air oxidation and then dialysis to remove urea restored the protein to its original functionality. (ii) Removing urea by dialysis and then allowing air oxidation of the denatured protein restored the protein to its original functionality. (iii) Denaturing the protein with both urea and β-mercaptoethanol yielded an inactive protein. (iv) Protein folding is determined by its primary sequence.arrow_forwardc) A lysine residue in the active site of UstD is involved in forming a covalent Schiff base linkage with the PLP cofactor. In order for this linkage to form, the lysine residue must be in its deprotonated form. Draw the structure of the R group of lysine in this neutral form. What is the ratio of the concentration of this form to that of its conjugate acid form at neutral pH? ( )arrow_forward= A different Fab fragment binds to lysozyme with a dissociation constant of Ka 10-6 M. A 1 nM (10-9 M) solution of lysozyme is treated with increasing concentrations of this Fab fragment. At what concentration of added Fab will half of the lysozyme be bound to this Fab? [F] = ab Marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Enzyme Kinetics; Author: MIT OpenCourseWare;https://www.youtube.com/watch?v=FXWZr3mscUo;License: Standard Youtube License