
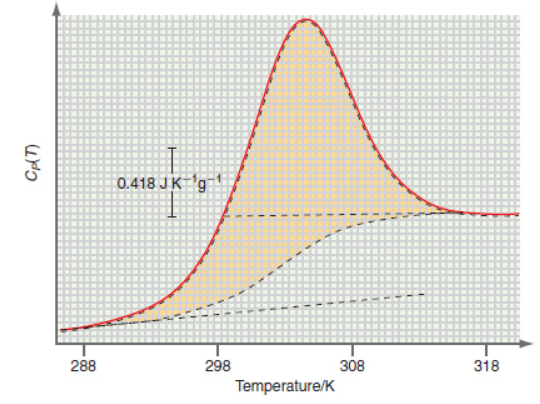
The following data are a DSC scan of a solution of a T4 lysozyme mutant. From the data determine
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Thermodynamics, Statistical Thermodynamics, & Kinetics
- Benzoic acid, C6H5COOH, is a common standard used in bomb calorimeters, which maintain a constant volume. If 1.20 g of benzoic acid gives off 31, 723 J of energy when burned in the presence of excess oxygen and in a water bath having a temperature of 24.6 C, calculate q, w, H, and U for the reaction.arrow_forwardYou did an experiment in which you found that 59.8 J was required to raise the temperature of 25.0 g of ethylene glycol (a compound used as antifreeze in automobile engines) by 1.00 K. Calculate the specific heat capacity of ethylene glycol from these data.arrow_forwardWhat are the two ways that a final chemical state of a system can be more probable than its initial state?arrow_forward
- Would the amount of heat absorbed by the dissolution in Example 5.6 appear greater, lesser, or remain the same if the heat capacity of the calorimeter were taken into account? Explain your answer.arrow_forwardThe enthalpy of combustion of diamond is -395.4 kJ/mol. C s, dia O2 g CO2 g Determine the fH of C s, dia.arrow_forwardDetermine the standard Gibbs free energy change, rG, for the reactions of liquid methanol, of CO(g), and ofethyne, C2H2(g), with oxygen gas to form gaseous carbondioxide and (if hydrogen is present) liquid water at298 K. Use your calculations to decide which of thesesubstances are kinetically stable and which are thermodynamically stable: CH3OH(), CO(g), C2H9(g), CO2(g),H2O().arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





