
Interpretation:
he method by which cathode rays are used to generate television and computer monitor images needs to be described.
Concept introduction:
In the cathode ray tube, a flow of electron in a strain line fall down on the anode just like a condensed beam and speed up the anode. The high speed beam of electrons are passed through a vacuum tube and hit at the end of the tube. The whole process is very fast and created a clear image on screen.
Answer to Problem 122A
The cathode ray tube (CRT) is very useful to produce the images on the television screen and computer screen with the flow of high speed of electron in a vacuum tube.
Explanation of Solution
In general the cathode ray tube is special type of vacuum tube, where images are created, when the electronic beam strikes on the phosphorescent surface.
The high speed electronic beam passed with very high velocity which is a thermionic emission. And the created images controlled by controlling the position of the electron that are fall down on the screen. The screen is coated with material that emits light when a beam of electron struck by screen.
The CRT TV work by movement of high speed of electron it is a very fast process to produce an images on the screen.
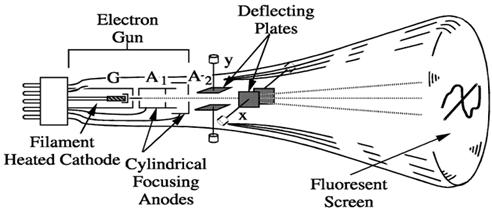
The main important parts of a cathode ray tube are as follows-
- Electron gun.
- Deflecting plates.
- Cylindrical focusing anode.
- Fluorescent screen.
Thus, the cathode ray tube TV and computer are very useful. It produce image on the screen just in few second, with high speed of the electron.
Chapter 4 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Campbell Essential Biology (7th Edition)
The Cosmic Perspective (8th Edition)
Campbell Biology (11th Edition)
Anatomy & Physiology (6th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Microbiology: An Introduction
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forward
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardOnly 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve itarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





