
Concept explainers
(a)
Interpretation: The given unbalanced reaction is to be balanced.
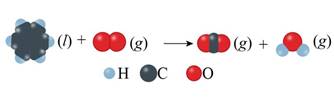
Concept introduction: Unbalanced chemical equations just reveal the species that are a part of the reaction. However, it doesn’t give any information regarding their relative amounts needed to satisfy law of mass conservation. This is reason why equations must actually be balanced. Balance reactions are characterized by balanced mass along with charge on both sides.
(a)
Answer to Problem 67E
The balanced equation is shown below.
Explanation of Solution
The given unbalanced equation is shown below.
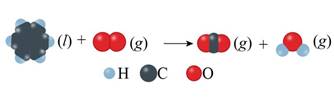
Figure 1
The given unbalanced equation using chemical formula of the species interpreted from the above figure is shown below.
In order to balance any equation, equalize the atoms of every element on reactant’s and product’s side.
The number of atoms of
Similarly, the number of atoms of
The number of atoms of
In order to obtain whole number stoichiometric coefficients, multiply all stoichiometric coefficients by
Since, the number of atoms of each element is equal on both sides, therefore the above equation is balanced.
(b)
Interpretation: The given unbalanced reaction is to be balanced.
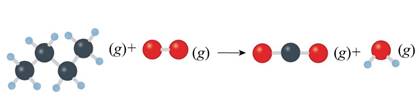
Concept introduction: Unbalanced chemical equations just reveal the species that are a part of the reaction. However, it doesn’t give any information regarding their relative amounts needed to satisfy low of mass conservation. This is reason why equations must actually be balanced. Balance reactions are characterized by balanced mass along with charge on both sides.
(b)
Answer to Problem 67E
The balanced equation is shown below.
Explanation of Solution
The given unbalanced equation is shown below.
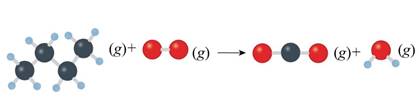
Figure 2
The given unbalanced equation using chemical formula of the species interpreted from the above figure is shown below.
In order to balance any equation, equalize the atoms of every element on reactant’s and product’s side.
The number of atoms of
Similarly, the number of atoms of
The number of atoms of
In order to obtain whole number stoichiometric coefficients, multiply all stoichiometric coefficients by
Since, the number of atoms of each element is equal on both sides, therefore the above equation is balanced.
(c)
Interpretation: The reaction
Concept introduction: Unbalanced chemical equations just reveal the species that are a part of the reaction. However, it doesn’t give any information regarding their relative amounts needed to satisfy low of mass conservation. This is reason why equations must actually be balanced. Balance reactions are characterized by balanced mass along with charge on both sides.
(c)
Answer to Problem 67E
The balanced equation is shown below.
Explanation of Solution
The given unbalanced equation is shown below.
In order to balance any equation, equalize the atoms of every element on reactant’s and product’s side.
The number of atoms of
Similarly, the number of atoms of
The number of atoms of
Since, the number of atoms of each element is equal on both sides, therefore the above equation is balanced.
(d)
Interpretation: The reaction
Concept introduction: Unbalanced chemical equations just reveal the species that are a part of the reaction. However, it doesn’t give any information regarding their relative amounts needed to satisfy low of mass conservation. This is reason why equations must actually be balanced. Balance reactions are characterized by balanced mass along with charge on both sides.
(d)
Answer to Problem 67E
The balanced equation is shown below.
Explanation of Solution
The given unbalanced equation is shown below.
In order to balance any equation, equalize the atoms of every element on reactant’s and product’s side.
The number of atoms of
Similarly, the number of atoms of
Since, the number of atoms of each element is equal on both sides, therefore the above equation is balanced.
(e)
Interpretation: The reaction
Concept introduction: Unbalanced chemical equations just reveal the species that are a part of the reaction. However, it doesn’t give any information regarding their relative amounts needed to satisfy low of mass conservation. This is reason why equations must actually be balanced. Balance reactions are characterized by balanced mass along with charge on both sides.
(e)
Answer to Problem 67E
The balanced equation is shown below.
Explanation of Solution
The given unbalanced equation is shown below.
In order to balance any equation, equalize the atoms of every element on reactant’s and product’s side.
The number of atoms of
Similarly, the number of atoms of
Since, the number of atoms of each element is equal on both sides, therefore the above equation is balanced.
Want to see more full solutions like this?
Chapter 3 Solutions
Chemical Principles
- (a) Butane gas, C4H10, can burn completely in air [use O2(g) as the other reactant] to give carbon dioxide gas and water vapor. Write a balanced equation for this combustion reaction. (b) Write a balanced chemical equation for the complete combustion of C3H7BO3, a gasoline additive. The products of combustion are CO2(g), H2O(g), and B2O3(s).arrow_forwardEthanol, C2H5OH, is a gasoline additive that can be produced by fermentation of glucose. C6H12O62C2H5OH+2CO2 (a) Calculate the mass (g) of ethanol produced by the fermentation of 1.000 lb glucose. (b) Gasohol is a mixture of 10.00 mL ethanol per 90.00 mL gasoline. Calculate the mass (in g) of glucose required to produce the ethanol in 1.00 gal gasohol. Density of ethanol = 0.785 g/mL. (c) By 2022, the U. S. Energy Independence and Security Act calls for annual production of 3.6 1010 gal of ethanol, no more than 40% of it produced by fermentation of corn. Fermentation of 1 ton (2.2 103 lb) of corn yields approximately 106 gal of ethanol. The average corn yield in the United States is about 2.1 105 lb per 1.0 105 m2. Calculate the acreage (in m2) required to raise corn solely for ethanol production in 2022 in the United States.arrow_forwardThe carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by reaction with lithium hydroxide 2LiOH(s)+CO2(g)Li2CO3(s)+H2O(l) Estimate the grams of lithium hydroxide required per astronaut per day. Assume that each astronaut requires 2.50 103 kcal of energy per day. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 2.50 103 kcal of heat, calculate the amount of CO2 produced and hence the amount of LiOH required. The H for glucose(s) is 1273 kJ/mol.arrow_forward
- Acetone, (CH3)2CO, is an important industrial compound. Although its toxicity is relatively low, workers using it must be careful to avoid flames and sparks because this compound burns readily in air. Write the balanced equation for the combustion of acetone.arrow_forward4.8 In an experiment carried out at very low pressure, 13x1015 molecules of H2 are reacted with acetylene, C2H2, to form ethane, C2H6, on the surface of a catalyst. Write a balanced chemical equation for this reaction. How many molecules of acetylene are consumed?arrow_forwardOxidation of 1.00 g of carbon monoxide, CO, produces 1.57 g of carbon dioxide, CO2. How many grams of oxygen were required in this reaction?arrow_forward
- 4.106 An ore sample with a mass of 670 kg contains 27.7% magnesium carbonate, MgCO3. If all of the magnesium carbonate in this ore sample is decomposed to form carbon dioxide, describe how to determine what mass of CO2 is evolved during the process.arrow_forwardCarbon dioxide from the atmosphere weathers, or dissolves, limestone (CaCO3) by the reaction CaCO3(s)+CO2(g)+H2O(l)Ca2(aq)+2HCO3(aq) Obtain H for this reaction. See Table 6.2 for the data.arrow_forwardNitric acid is produced commercially by the Ostwald process, represented by the following equations: 4NH3(g)+5O24NO(g)+6H2O(g)2NO(g)+O2(g)2NO2(g)3NO2(g)+H2O(l)2HNO3(aq)+NO(g) What mass of NH3 must be used to produce 1.0 106 kg HNO3 by the Ostwald process? Assume 100% yield in each reaction, and assume that the NO produced in the third step is not recycled.arrow_forward
- Iron oxide ores, commonly a mixture of FeO and Fe2O3, are given the general formula Fe3O4. They yield elemental iron when heated to a very high temperature with either carbon monoxide or elemental hydrogen. Balance the following equations for these processes: Fe3O4(s)+H2(g)Fe(s)+H2O(g)Fe3O4(s)+CO(g)Fe(s)+CO2(g)arrow_forwardDisulfur dichloride, S2Cl2, is used to vulcanize rubber. It can be made by treating molten sulfur with gaseous chlorine. S8() + 4 Cl2(g) 4 S2Cl2(g) Complete this table of reaction quantities for the production of 103.5 g S2Cl2.arrow_forwardlist at least three quantities that must be conserved in chemical reactions.arrow_forward

 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





