
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 3.2P
(a) Classify the carbon atoms in each compound as
[1] 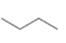 [2]
[2] [3]
[3]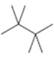 [4]
[4]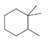
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which two compounds are identical?
HINT: To answer this, you will need to NAME each one correctly.
A) Compounds 1 and 3
Compound 1
Compound 2
B) Compounds 2 and 4
C) Compounds 1 and 2
D) Compounds 2 and 3
Compound 3
Compound 4
pter 4 Practice
4.15 Polycyclic Systems
Identify the relationship between the following compounds.
& A
Br
H
H
constitutional isomers
O different compounds
O stereoisomers
O identical
Hint
Br
Save for Later
MacBook Air
5) Use compounds X, Y and Z (shown below) to answer the following questions.
H.
H3C"
"CH3
ОН
CH3
compound X
compound Y
compound Z
a) Is compound X classified as cis, trans or neither?
b) Is compound Y classified as E, Z or neither?
c) Is compound Z classified as R, S or neither?
d) Are compounds X and Y constitutional isomers of each other? (YES or NO)
e) Are compounds Y and Z constitutional isomers of each other? (YES or NO)
f) Classify compound Y as a primary, secondary or tertiary alcohol.
g) Which compound(s) is(are) chiral?
h) Give the IUPAC name for compound Z, omitting the absolute configuration (R or S) designation.
i) In the indicated spaces below, draw stereoisomers of compounds X, Y and Z.
stereoisomer of compound X
stereoisomer of compound Y
stereoisomer of compound Z
Chapter 3 Solutions
Organic Chemistry
Ch. 3 - Prob. 3.1PCh. 3 - (a) Classify the carbon atoms in each compound as...Ch. 3 - Problem 3.3 Classify a carbon atom by the number...Ch. 3 - Classify each alkyl halide and alcohol as , or...Ch. 3 - Prob. 3.5PCh. 3 - Prob. 3.6PCh. 3 - Draw the structure of a compound of molecular...Ch. 3 - Prob. 3.8PCh. 3 - Prob. 3.9PCh. 3 - Draw the structure of a compound fitting each...
Ch. 3 - Draw structures that fit each description and name...Ch. 3 - What types of intermolecular forces are present in...Ch. 3 - Which compound in each pair has the higher boiling...Ch. 3 - Explain why the boiling point of propanamide, is...Ch. 3 - Predict which compound in each pair has the higher...Ch. 3 - Prob. 3.16PCh. 3 - Which compounds are water soluble? a. b. c.Ch. 3 - a Label the hydrophobic and hydrophilic portions...Ch. 3 - Prob. 3.19PCh. 3 - Prob. 3.20PCh. 3 - Prob. 3.21PCh. 3 - Prob. 3.22PCh. 3 - Problem 3.23 (a) What types of intermolecular...Ch. 3 - Prob. 3.24PCh. 3 - Prob. 3.25PCh. 3 - Problem 3.26 Label the electrophilic and...Ch. 3 - Problem 3.27 Considering only electron density,...Ch. 3 - Prob. 3.28PCh. 3 - 3.29
Identify the functional groups in the...Ch. 3 - Prob. 3.30PCh. 3 - 3.31 For each alkane: (a) classify each carbon...Ch. 3 - 3.32 Identify the functional groups in each...Ch. 3 - 3.33 Identify each functional group located in the...Ch. 3 - 3.34 (a)Identify the functional groups in...Ch. 3 - Draw seven constitutional isomers with molecular...Ch. 3 - Prob. 3.36PCh. 3 - Prob. 3.37PCh. 3 - Prob. 3.38PCh. 3 - Intramolecular force of attraction are often...Ch. 3 - 3.40 (a) Draw four compounds with molecular...Ch. 3 - 3.41 Rank the compounds in each group in order of...Ch. 3 - Explain why CH3CH2NHCH3 has higher boiling point...Ch. 3 - Prob. 3.43PCh. 3 - 3.44 Rank the following compounds in order of...Ch. 3 - Prob. 3.45PCh. 3 - 3.46 Rank the following compounds in order of...Ch. 3 - 3.47 Which of the following molecules can hydrogen...Ch. 3 - 3.48 Explain why diethylether and have similar...Ch. 3 - Prob. 3.49PCh. 3 - 3.50 Predict the solubility of each of the...Ch. 3 - Prob. 3.51PCh. 3 - Prob. 3.52PCh. 3 - 3.53 THC is the active component in marijuana, and...Ch. 3 - Prob. 3.54PCh. 3 - Prob. 3.55PCh. 3 - 3.56 Label the electrophilic and nucleophilic...Ch. 3 - 3.57 By using only electron density arguments,...Ch. 3 - 3.58 The composition of a cell membrane is not...Ch. 3 - Prob. 3.59PCh. 3 - 3.60 Quinapril (trade name Accupril) is a drug...Ch. 3 - 3.61 Answer each question about oxycodone, a...Ch. 3 - Prob. 3.62PCh. 3 - Prob. 3.63PCh. 3 - 3.64 Explain why A is less water soluble than B,...Ch. 3 - 3.65 Recall from section 1.10B that there is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) Are compounds B–D identical to or an isomer of A? (b) Give the IUPAC name for A.arrow_forwardCI CI CI HO Et A Give the IUPAC name for the four compounds illustrated above. (Don't forget cis/trans where needed.) Compound A Compound B Compound Carrow_forwardWhich compounds (B–F) are identical to A? (b) Which compounds (B–F) represent an isomer of A?arrow_forward
- How are the following two compounds related to each other? H H3C KD H3C CH3 a) They are constitutional isomers of each other. O b) O c) H CH3 d) They are enantiomers of each other. They are stereoisomers of each other. They are functional isomers of each other.arrow_forwardConvert the following molecules into condensed formulas and IUPAC names.arrow_forwardChondrocole A is a marine natural product isolated from red seaweed that grows in regions of heavy surf in the Pacific Ocean. (a) Predict the solubility of chondrocole A in water and CH2Cl2. (b) Locate the stereogenic centers and label each as R or S. (c) Draw a stereoisomer and a constitutional isomer of chondrocole A.arrow_forward
- The cis ketone A is isomerized to a trans ketone B with aqueous NaOH. A similar isomerization does not occur with ketone C. (a) Draw the structure of B using a chair cyclohexane. (b) Label the substituents in C as cis or trans, and explain the difference in reactivity.arrow_forwardAnswer each question using the ball-and-stick model of compound A. Draw a stereoisomer for A and give its IUPAC name.Draw a constitutional isomer that contains an OH group and give itsIUPAC name.arrow_forwardOut of CH3—NH2 and (CH3)3N, which one has higher boiling point?arrow_forward
- For the following molecule indicate what class of compounds each arrow is pointing atarrow_forwardDraw the structure of all compounds that f t the following descriptions. a. Five constitutional isomers having the molecular formula C4H8. b. Nine constitutional isomers having the molecular formula C7H16. c. Twelve constitutional isomers having the molecular formula C6H12 and containing one ringarrow_forwardList each set of a compound in order of increasing boiling point.(1) octane, (CH3)3C¬C(CH3)3, and CH3CH2C(CH3)2CH2CH2CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY