
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 3.27P
Considering only electron density, state whether the following reactions will occur.
a. 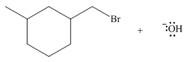 c.
c. 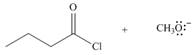
b. 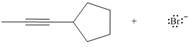 d.
d. 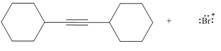
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
23.
Give detailed Solution
show-all-working-explaining-detailly-each-step
Answer should be typewritten with a computer keyboard.
Chapter 3 Solutions
Organic Chemistry
Ch. 3 - Prob. 3.1PCh. 3 - (a) Classify the carbon atoms in each compound as...Ch. 3 - Problem 3.3 Classify a carbon atom by the number...Ch. 3 - Classify each alkyl halide and alcohol as , or...Ch. 3 - Prob. 3.5PCh. 3 - Prob. 3.6PCh. 3 - Draw the structure of a compound of molecular...Ch. 3 - Prob. 3.8PCh. 3 - Prob. 3.9PCh. 3 - Draw the structure of a compound fitting each...
Ch. 3 - Draw structures that fit each description and name...Ch. 3 - What types of intermolecular forces are present in...Ch. 3 - Which compound in each pair has the higher boiling...Ch. 3 - Explain why the boiling point of propanamide, is...Ch. 3 - Predict which compound in each pair has the higher...Ch. 3 - Prob. 3.16PCh. 3 - Which compounds are water soluble? a. b. c.Ch. 3 - a Label the hydrophobic and hydrophilic portions...Ch. 3 - Prob. 3.19PCh. 3 - Prob. 3.20PCh. 3 - Prob. 3.21PCh. 3 - Prob. 3.22PCh. 3 - Problem 3.23 (a) What types of intermolecular...Ch. 3 - Prob. 3.24PCh. 3 - Prob. 3.25PCh. 3 - Problem 3.26 Label the electrophilic and...Ch. 3 - Problem 3.27 Considering only electron density,...Ch. 3 - Prob. 3.28PCh. 3 - 3.29
Identify the functional groups in the...Ch. 3 - Prob. 3.30PCh. 3 - 3.31 For each alkane: (a) classify each carbon...Ch. 3 - 3.32 Identify the functional groups in each...Ch. 3 - 3.33 Identify each functional group located in the...Ch. 3 - 3.34 (a)Identify the functional groups in...Ch. 3 - Draw seven constitutional isomers with molecular...Ch. 3 - Prob. 3.36PCh. 3 - Prob. 3.37PCh. 3 - Prob. 3.38PCh. 3 - Intramolecular force of attraction are often...Ch. 3 - 3.40 (a) Draw four compounds with molecular...Ch. 3 - 3.41 Rank the compounds in each group in order of...Ch. 3 - Explain why CH3CH2NHCH3 has higher boiling point...Ch. 3 - Prob. 3.43PCh. 3 - 3.44 Rank the following compounds in order of...Ch. 3 - Prob. 3.45PCh. 3 - 3.46 Rank the following compounds in order of...Ch. 3 - 3.47 Which of the following molecules can hydrogen...Ch. 3 - 3.48 Explain why diethylether and have similar...Ch. 3 - Prob. 3.49PCh. 3 - 3.50 Predict the solubility of each of the...Ch. 3 - Prob. 3.51PCh. 3 - Prob. 3.52PCh. 3 - 3.53 THC is the active component in marijuana, and...Ch. 3 - Prob. 3.54PCh. 3 - Prob. 3.55PCh. 3 - 3.56 Label the electrophilic and nucleophilic...Ch. 3 - 3.57 By using only electron density arguments,...Ch. 3 - 3.58 The composition of a cell membrane is not...Ch. 3 - Prob. 3.59PCh. 3 - 3.60 Quinapril (trade name Accupril) is a drug...Ch. 3 - 3.61 Answer each question about oxycodone, a...Ch. 3 - Prob. 3.62PCh. 3 - Prob. 3.63PCh. 3 - 3.64 Explain why A is less water soluble than B,...Ch. 3 - 3.65 Recall from section 1.10B that there is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What will happen if the solution of precipitated sulfur U.S.P. is made acidic? Write the equation as well.arrow_forward6. Which of the following reactants would NOT be expected to do an acid/base reaction? A. B. NH NAOH + CH3-NH2 HO, C. D. Mg(OH)2 HBr +. HO. NH2arrow_forwardHow many milliter of 0.200M NaOH will be required to neutralize 75.0mL of 0.215M H2SO4? a.161 b.323 c.323 d.430arrow_forward
- Metals tend to form cations and almost have positive oxidation numbers a. Trueb. Falsearrow_forwardHow Many milliliters of 0.105 M NaOH are reqiured to neutralize exactly 14.2 mL of 0.141 M H3PO4arrow_forward7. Cite at least 3 examples of bases. b. a. C. 8. What is a substance that is capable of donating a proton? 9. What is a substance that is capable of accepting a proton? 10. Water is amphoteric, which meansarrow_forward
- What best sums up Le Chatelier's principle is A. According to this, a system in balance tends to change in a way that releases tension while under stress. B. Le Chatelier's principle can be applied in several situations, such as when you blow air through a straw or dissolve an alka-seltzer pill in a glass of water. C. explains how acids are created and destroyed, and how anything can operate to raise the concentration. D. All of the abovearrow_forwardE' I с E 1. Phenylamine is an aromatic amine that is used in the manufacture of dyes. When absorbed through the skin it causes the Fe+2 in hemoglobin to become oxidized into Fe+3, resulting in the formation of methemoglobin which cannot bind to or transport oxygen. Phenylamine is soluble in water and acts as a weak base. a. a. CøHşNHz (aq) + H2O (l) = CHşNH;* (aq) + OH (aq) When you measure the concentrations of the ionized substances you find them to be: [C6H5NH₂] = 0.234 mol/L [C6H5NH3*] = 2.8 x 10³ mol/L [OH-]= 2.8 x 10³ mol/L If the K is 4.27 x 10-10, is the reaction at equilibrium? If not, which direction does it need to move (right or left) to reach equilibrium? Explain. At equilibrium the concentrations of the ionized substances are: [C6H5NH₂] = 0.0537 mol/L [C6H5NH3*] =4.79 x 10€ mol/L [OH-]= 4.79 x 10¹ mol/L If this reaction is taking place in a 2.0L container, and 1.5 moles of phenylamine were added to the reaction, what will the new concentrations of the three ionic species…arrow_forwardPredict whether each compound would form an acid or a base in a reaction with water. c. sulfur trioxidearrow_forward
- Hyrochloric acid is a strong acid that may be effective for neutralizing a wide range of bases. However, it may not be the best choice for neutralization. What problems may be encountered when using a strong acid for neutralizing a base?arrow_forward2. Electrolytes: Definitions. Answers provided in lecture notes. A. Strong Electrolyte B. Weak electrolyte C. Nonelectrolyte D. Give seven examples of strong acidsarrow_forward6. Which of the following reactants would NOT be expected to do an acid/base reaction? A. C. ΝΗ + OH + Mg(OH)2 OH B. D. NaOH + CH3-NH2 HBr + NH₂arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY