(a)
To determine:
Whether a H bond will be formed in a reaction between H3C-CH=O and H2O with the help of a figure.
Introduction:
(b)
To determine:
With the help of diagrams if the molecules Cl2, NH3 and CH4 will be polar or not.
Introduction:
When atoms combine to form molecules, they form chemical bonds by sharing, donating or accepting electrons. In case of sharing, the combining atoms may or may not share the electrons equally. If one of the atoms is more electronegative, it pulls the shared electron cloud towards itself and acquires negative charge. The other atom thus acquires positive charge. Such a molecule is called polar.
(c)
To determine:
The pH of a solution with a H+ concentration of 0.00001 M.
Introduction:
Dissociation of water results in generation of H+ and OH- ions. However, these ions are equal in number. Acids are compounds that release H+ in solution and bases are compounds that release OH- in solution. The pH scale expresses the concentration of H+ ions in a solution. pH less than 7 is indicative of acidic solution and pH above 7 indicates a basic (alkaline) solution. pH 7 is an indicator of a neutral solution.
(d)
To determine:
The pH of a solution with a OH- concentration of 0.00001 M.
Introduction:
Dissociation of water results in generation of H+ and OH- ions. However, these ions are equal in number. Acids are compounds that release H+ in solution and bases are compounds that release OH- in solution. The pH scale expresses the concentration of H+ ions in a solution. pH less than 7 is indicative of acidic solution and pH above 7 indicates a basic (alkaline) solution. pH 7 is an indicator of a neutral solution.
(e)
To determine:
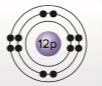
The kinds of bonds that magnesium can make based on the diagram of its atomic structure.
Introduction:
Atoms combine with other atoms by making chemical bonds with them. These bonds can be made by either sharing, donating or accepting electrons. The number of electrons in the outer-most orbital of an atom determine its tendency to form bonds. These electrons are called valence electrons. Chemical bonds are formed in a way that the outer-most orbital is completely filled with electrons.
(f)
To determine:
The kind of ion that Magnesium would make, depending on its valence.
Introduction:
Atoms combine with other atoms by making chemical bonds with them. These bonds can be made by either sharing, donating or accepting electrons. The number of electrons in the outer-most orbital of an atom determine its tendency to form bonds. These electrons are called valence electrons. Chemical bonds are formed in a way that the outer-most orbital is completely filled with electrons.
Explanation:
Looking at the atomic structure of Magnesium, it is observed that the 12 electrons are distributed is different orbital as 2, 8 and 2 electrons. The outer-most orbital has 2 electrons. Thus, Mg forms bonds by giving up its two valence electrons to atoms that may accept them. Thus, Mg loses 2 electrons to become positively charged ion Mg2+. Positively charged ions are also known as cations.
Conclusion:
Magnesium loses two electrons from its outer-most orbital to gain positive charge on its ion.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Foundations in Microbiology
- A solution contains 0.25 M Ni(NO3)2 and 0.25 M Cu(NO3)2. A. Can the metal ions be separated by slowly adding Na2CO3 aq solution? Assume that for successful separation 99% of the metal ion must be precipitated before the other metal ion begins to precipitate, and assume no volume change on addition of Na2CO3 solution. Ksp of CuCO3 = 1.4 x10-10 Ksp of NiCO3 = 1.42X 10-7 B. If you need to redissolve the salt that was first precipitated, what pH will you use and why ? Write your answers in template provided. Upload your step by step solution for A.arrow_forwardA mixture of proteins contains four different polypeptides, all in ~equal concentration, in solution with the following properties: Protein Molecular Mass (kDa) Isoelectric point A 45 4.5 B 77 6.0 C 28 4.1 D 14 10.7 A fraction of the protein solution is applied to a strong cation exchange column using a buffer at pH 8.0 with increasing [NaCl] from 0.05 M – 1.0 M. The chromatogram is shown below: 1. Based on the data presented, which of the following statements is true: Peak #1 is protein D Peak #4 is protein C Peak #3 is protein A Peak #4 is protein D Peak #2 is protein B 2. Since you know that the proteins are all present in approximately equal concentrations, the different relative peak areas tell you that: There are more neutral amino acids in protein #4…arrow_forwardThe ionization of p-nitrophenol is shown below (pKa = 7.0): a. Identify the weak acid and conjugate base. b. At pH 7, what are the relative concentrations of ionized and un-ionized p-nitrophenol? c. If enough concentrated hydrochloric acid is added to a solution of p-nitrophenol to lower the pH from 7 to 5, what will happen to the relative concentrations of the ionized and un-ionized forms? d. Ionized p-nitrophenol has a yellow color, while the un-ionized form is colorless. The yellow color can be measured using a spectrophotometer at 400nm. In order to determine the total amount of p-nitrophenol in a solution, would you perform the spectrophotometer reading at an acidic or basic pH? Clearly explain why? e. A solution of p-nitrophenol at pH 7.95 was found to have an A400 of 0.255 . What is the total concentration (in µM) of p-nitrophenol (ionized plus un-ionized) in the solution? The molar extinction coefficient of p-nitrophenol is 18,500 M-1cm-1 and the pKa is 7.arrow_forward
- Work out the following problems: a. What is the pH of a solution with a concentration of0.00001 moles/ml (M) of OH−?b. Draw the atomic structure of magnesium and predict what kindsof bonds it will make.c. What kind of ion would you expect magnesium to make on thebasis of its valence?arrow_forwardCalculate the concentration of Y4-ion in 0.0100 M EDTA solution at pH 6. for pH 6, α4 = 2,2 x 10-5arrow_forwardCalculate the normality of a solution that contains 4.5 g of (COOH)2 in 3000 mL of solution? (Assume the (COOH)2 is to be completely neutralized in an acid-base * reaction.) 0.033 N O 0.33 N O 0.166 N O 0.0166 N 0.45 N O 0.045 N 000.0 OOarrow_forward
- The pH of a fruit juice is 4.8. Find the hydronium ion concentration, [H3O*], 0 of the juice. Use the formula pH = -log [H3O*]. ... The hydronium ion concentration [H3O*] is approximately moles per liter. (Use scientific notation. Use the multiplication symbol in the math palette as needed. Round to the nearest tenth as needed.)arrow_forwardWhat mass of sodium glycolate (NaC2H3O3) should be added to 400.0 mL of 1.00 M glycolic acid to produce a buffer solution with a pH of 4.00? Ka = 1.47 x 10-4. Please indicate the full solutions.arrow_forward3.) A mixture of amino acids was subjected to paper chromatography with six amino acid standards in order to determine its composition. After visualization using ninhydrin, the measured distances were as follows: A B с D E F G a.) What is the Rf value of each amino acid observed? X Distances: Solvent 10.3cm A 4.50cm B - 1.32cm C - 6.60cm D - 5.20cm E - 3.52cm F 8.70cm G - 10.26cm X1 - 9.72cm X2 - 4.3cm X3 7.1cm - b.) What are the possible components of the amino acid mixture? Answer can be up to two amino acids per component.arrow_forward
- This is your promised calculation for unsaturation. An oil (8.36g, MW=400g/mol) requires 38.95mL of 2.683M bromine to completely react with all of the double bonds of the lipid. a. How many moles of lipid do you have? b. How many moles of bromine d'id you use? C. How many double bu nds are present in this lipidi d. Propose a structure for the triglyceride that could give the results in part c. There are many, many correct answers here. Again, NO STICKS!arrow_forwardCalculate the pH of a solution that is 0.05000 M NH4Cl and 0.0300 M NH3 (Kb for NH3 = 1.8 x 10-5)arrow_forwardYou need to make a protein buffer of: . • • 100 mM NaCl 25 mM Tris 8 5% w/v glycerol • 2 mM DTT Your stock solutions are: 5 M NaCl • 2 M Tris 8 • 70% w/v glycerol • DTT @ 154.25 g/mol How would you make a 1L protein buffer Solution? Show your work and describe.arrow_forward
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education





