
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2.9, Problem 2.5CIAP
(a)
Interpretation Introduction
Interpretation:
The higher energy should be identified.
Concept introduction:
The shorter the wavelength, the higher the energy, the longer the wavelength, the lower the energy.
Wave length region is shown in figure 1.
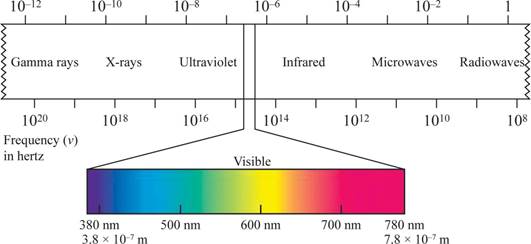
Figure 1
(b)
Interpretation Introduction
Interpretation:
The higher energy should be identified.
Concept introduction:
The shorter the wavelength, the higher the energy, the longer the wavelength, the lower the energy.
Wave length region is shown in figure 1.
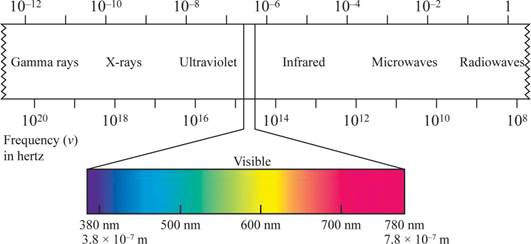
Figure 1
(c)
Interpretation Introduction
Interpretation:
The higher energy should be identified.
Concept introduction:
The shorter the wavelength, the higher the energy, the longer the wavelength, the lower the energy.
Wave length region is shown in figure 1.
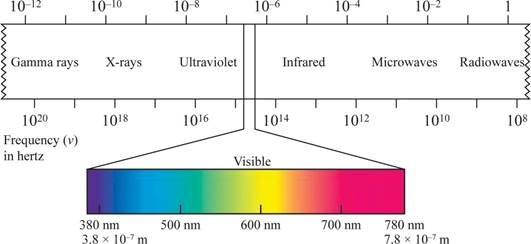
Figure 1
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Assume that you list the following types of electromagnetic radiation in order of increasing
wavelength: () the gamma rays produced by a radioactive nuclide used in medical imaging:
(ii) radiation from an FM radio station at 93.1 MHz on the dial; (iii) a radio signal from an AM
radio station at 680 kHz on the dial; (iv) the yellow light from sodium vapor streetlights; (v) the
red light of a light-emitting diode. Which one would be the second?
Lütfen birini seçin:
O a 680 kHz AM radio waves
O b. the red light
O c the yellow light
O d. 93.1 MHz FM radio waves
O e the gamma rays
Which of the following statements is true? 1) The shortest wavelength of light is the ultraviolet ray, which is the highest in energy. 2) The longest wavelength of light is the ultraviolet ray, which is the highest in energy 3) The shortest wavelength of light is the visible light, which is the highest in energy 4 ) The shortest wavelength of light is the infrared ray, which is the highest in energy. 5) The longest wavelength of light is the infrared raywhich is the highest in energy .
(A) how much energy is necessary to heat 7.0 kg of water from room temperature (20 degrees Celsius) to its boiling point? (Assume no energy loss)
(b) if electrical energy were used, how much would this cost at 45 cent per kWh?
Chapter 2 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 2.1 - Prob. 2.1CIAPCh. 2.1 - For the Kanji character in the lower portion of...Ch. 2.2 - Use the list inside the front cover to identify...Ch. 2.3 - Prob. 2.2PCh. 2.3 - Prob. 2.3PCh. 2.3 - Prob. 2.4PCh. 2.4 - Prob. 2.5PCh. 2.4 - Prob. 2.6PCh. 2.4 - Prob. 2.7PCh. 2.4 - Prob. 2.8P
Ch. 2.4 - Prob. 2.9PCh. 2.5 - Prob. 2.10PCh. 2.5 - Prob. 2.11PCh. 2.5 - Prob. 2.12PCh. 2.5 - Prob. 2.13KCPCh. 2.5 - Prob. 2.3CIAPCh. 2.5 - Prob. 2.4CIAPCh. 2.6 - Prob. 2.14PCh. 2.7 - Prob. 2.15PCh. 2.7 - Write electron configurations for the following...Ch. 2.7 - Prob. 2.17PCh. 2.7 - Identify the atom with the following...Ch. 2.8 - Prob. 2.19PCh. 2.8 - Prob. 2.20PCh. 2.8 - Prob. 2.21PCh. 2.8 - Prob. 2.22KCPCh. 2.9 - Prob. 2.23PCh. 2.9 - Write electron-dot symbols for radon, lead, xenon,...Ch. 2.9 - Prob. 2.25PCh. 2.9 - Prob. 2.5CIAPCh. 2.9 - Prob. 2.6CIAPCh. 2 - Where on the following outline of a periodic table...Ch. 2 - Is the element marked in red on the following...Ch. 2 - Prob. 2.28UKCCh. 2 - What atom has the following orbital-filling...Ch. 2 - Use the following orbital-filling diagram to show...Ch. 2 - What four fundamental assumptions about atoms and...Ch. 2 - How do atoms of different elements differ?Ch. 2 - Prob. 2.33APCh. 2 - Prob. 2.34APCh. 2 - Prob. 2.35APCh. 2 - Prob. 2.36APCh. 2 - How many O atoms of mass 15.99 amu are in 15.99 g...Ch. 2 - Prob. 2.38APCh. 2 - What are the names of the three subatomic...Ch. 2 - Prob. 2.40APCh. 2 - Prob. 2.41APCh. 2 - Prob. 2.42APCh. 2 - Which of the following symbols represent isotopes...Ch. 2 - Prob. 2.44APCh. 2 - Prob. 2.45APCh. 2 - Prob. 2.46APCh. 2 - One of the most widely used isotopes in medical...Ch. 2 - Prob. 2.48APCh. 2 - Prob. 2.49APCh. 2 - Prob. 2.50APCh. 2 - Prob. 2.51APCh. 2 - Prob. 2.52APCh. 2 - Prob. 2.53APCh. 2 - Prob. 2.54APCh. 2 - Prob. 2.55APCh. 2 - For (a) rubidium (b) tungsten, (c) germanium, and...Ch. 2 - For (a) calcium, (b) palladium, (c) carbon, and...Ch. 2 - Prob. 2.58APCh. 2 - Prob. 2.59APCh. 2 - Prob. 2.60APCh. 2 - Prob. 2.61APCh. 2 - Prob. 2.62APCh. 2 - Prob. 2.63APCh. 2 - Prob. 2.64APCh. 2 - Prob. 2.65APCh. 2 - Prob. 2.66APCh. 2 - Prob. 2.67APCh. 2 - Prob. 2.68APCh. 2 - Prob. 2.69APCh. 2 - Prob. 2.70APCh. 2 - Prob. 2.71APCh. 2 - Determine the number of unpaired electrons for...Ch. 2 - Without looking back in the text, write the...Ch. 2 - Prob. 2.74APCh. 2 - Prob. 2.75APCh. 2 - Prob. 2.76APCh. 2 - Prob. 2.77APCh. 2 - Prob. 2.78APCh. 2 - Using n for the number of the valence shell and...Ch. 2 - What elements in addition to helium make up the...Ch. 2 - Prob. 2.81CPCh. 2 - What is the atomic number of the yet-undiscovered...Ch. 2 - Give the number of electrons in each shell for...Ch. 2 - Identify the highest-energy occupied subshell in...Ch. 2 - Prob. 2.85CPCh. 2 - Prob. 2.86CPCh. 2 - Germanium, atomic number 32, is used in building...Ch. 2 - Prob. 2.88CPCh. 2 - Prob. 2.89CPCh. 2 - What is wrong with the following electron...Ch. 2 - Prob. 2.91CPCh. 2 - Prob. 2.92CPCh. 2 - Prob. 2.93CPCh. 2 - Prob. 2.94CPCh. 2 - Prob. 2.95GPCh. 2 - Prob. 2.96GPCh. 2 - Prob. 2.97GPCh. 2 - Look again at the trends illustrated in Figures...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) How much time is needed to measure the kinetic energy of an electron whose speed is 10.0 m/s with an uncertainty of no more than 0.100 percent? How far will the electron have traveled in this period of time? (b) Make the same calculations for a 1.00-g insect whose speed is the same. What do these sets of figures indicate?arrow_forwardThe magnesium electron spectrum has a line at 266.8 nm. Does it have a greater speed in a vacuum (an area without an atom or particle) than does red light with a wavelength of 652 nm? Why or why not?arrow_forwardA slice of pizza has 500 kcal. If we could burn the pizza and useall the heat to warm a 50-L container of cold water, what wouldbe the approximate increase in the temperature of the water?(Note: A liter of cold water weighs about 1 kg.)(A) 50°C (B) 5°C(C) 100°C (D) 10°Carrow_forward
- At the beginning of an experiment, a scientist has 268 grams of radioactive goo. After 180 minutes, her sample has decayed to 4.1875 grams.What is the half-life of the goo in minutes? Find a formula for G(t)G(t), the amount of goo remaining at time tt. How many grams of goo will remain after 32 minutes?arrow_forwardIf a hospital were storing radioisotopes, what is the minimum containment needed to protect against:(a) cobalt-60 (a strong γ emitter used for irradiation)(b) molybdenum-99 (a beta emitter used to produce technetium-99 for imaging)arrow_forwardWhich of the following types of photons (packets of pure electromagnetic energy) have the highest energy level? visible light infrared microwaves ultra-violetarrow_forward
- In your own words define what is Dark matter?arrow_forwardIf there is 10 μmol of the radioactive isotope 32P (half-life 14 days) at t = 0, how much 32P will remain at (a) 7 days, (b) 14 days, (c) 21 days, and (d) 70 days?arrow_forward(I) Why the physicochemical changes in liquid water caused by radiation is the key to understanding the biological effects of radiation? Please give a short, one-sentence explanation. (II) Name the three key stages of the physicochemical changes produced in liquid water due to radiation. (III) Briefly describe the three key stages. A one- or two-sentence explanation for each of the three key stages would be sufficient.arrow_forward
- If a particle in an electric field E experiences a force F, what also must be true?A)The particle has a nonzero charge.B) The particle is positively charged.C) The particle is negatively charged.D) The particle is moving.arrow_forwardThis is about Beer-Lambert’s Law and UV-vis Spectroscopy: 1) How does a solution interact with light?2) How are absorbance and transmittance related? 3) How do you make an absorbance spectrum?arrow_forward(4) The normal melting point of gold is 1064.5 °C and its boiling point is 2660 °C. (a) Convert these two values to the Fahrenheit and Kelvin scales. (b) Find the difference between those two values in Celsius. (c) Repeat (b) using the Kelvin scale.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning

Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning
What is cancer? What causes cancer and how is it treated? *UPDATE*; Author: Cancer Treatment Centers of America - CTCA;https://www.youtube.com/watch?v=_N1Sk3aiSCE;License: Standard Youtube License