
Concept explainers
(a)
Interpretation:
The explanation for alanine which has different optical rotation in water,
Concept introduction:
The amino acid is made of two
Answer to Problem 27.61AP
Each solvent rotates the alanine molecule to a different angle due to the formation of different complex formation and so it gives rise to different optical rotation in different solvents.
Explanation of Solution
The structure of alanine is shown below.
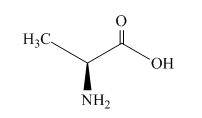
Figure 1
Alanine is an optically active compound. It rotates the plane of polarized light. The reaction of alanine with water,
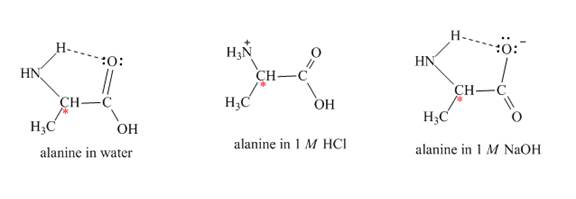
Figure 2
The optical rotation of alanine is measure by Circular Dichroism (CD). It involves circularly polarized light absorption. It measures the angle at which the plane-polarized light is rotated by the molecule. Each solvent rotates the alanine molecule to a different angle, due to this it gives rise to different optical rotation in different solvents.
The alanine has different optical rotation in water,
(b)
Interpretation:
The explanation for two known mono
Concept introduction:
The amino acid is made of two functional groups an amine group,
Answer to Problem 27.61AP
Due to the presence of two amine groups in lysine molecule. It may undergo acetylation reaction from either side and form mono
Explanation of Solution
The structure of amino acid lysine is shown below.
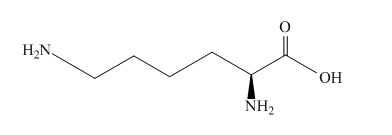
Figure 3
The structure of the lysine molecule contains two amine group one at the
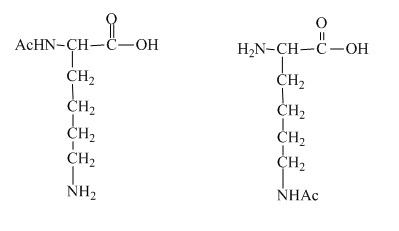
Figure 4
The two mono
(c)
Interpretation:
The explanation for fact that the peptide
Concept introduction:
The amino acid is made of two functional groups an amine group
Answer to Problem 27.61AP
The compound urea under basic conditions acts as a denaturation agent which breakdown the protein molecule bonding. Due to this, the peptide,
Explanation of Solution
The protein molecule is composed of four types of structure primary, secondary, tertiary and quarternary. The enzyme trypsin hydrolyzes the peptide,
The peptide
(d)
Interpretation:
The explanation for the peptide containing cysteine on reaction with
Concept introduction:
The amino acid is made of two functional groups an amine group,
Answer to Problem 27.61AP
The generation of lysine type molecule at the end of the reaction which is cleaved by trypsin enzyme. Due to this, the peptide containing cysteine on reaction with
Explanation of Solution
The structure of the cysteine molecule is shown below.
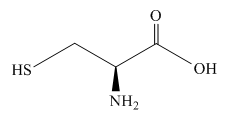
Figure 5
The same molecule in peptides exists as a disulfide bond. The disulfide bond of cysteine in the protein molecule is shown below.
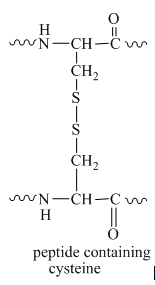
Figure 6
When this protein molecule with a disulfide bond is treated with
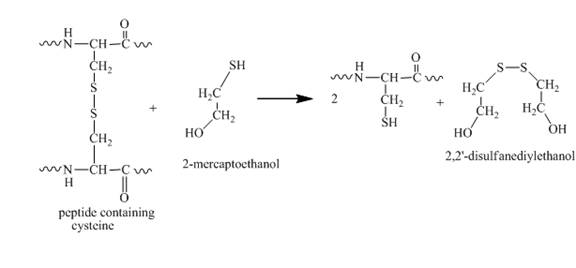
Figure 7
The thiol group is then reacted with the aziridine molecule which results in the formation of a lysine type molecule. This reaction is shown below.
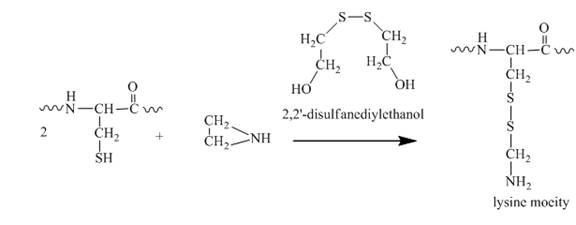
Figure 8
This lysine type molecule then reacts with trypsin enzyme which cleaves arginine and lysine molecule. Due to this, the trypsin enzyme reacts with the modified cysteine residues.
The peptide containing cysteine on reaction with
(e)
Interpretation:
The explanation for the formation of two separable methionine sulfoxides from the oxidation of
Concept introduction:
The amino acid is made of two functional groups an amine group,
Answer to Problem 27.61AP
The application of a certain amount of energy which converts one form to another form of structure. Due to this, the formation of two separable methionine sulfoxides from the oxidation of
Explanation of Solution
The structure of methionine is shown below.
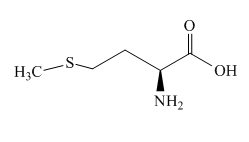
Figure 9
The resonance structure of sulfoxides with two different groups is shown below.
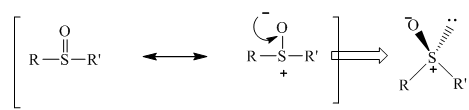
Figure 10
The conversion of one structure to another structure at room temperature requires a certain amount of energy. Therefore, on the application of that amount of energy, the two forms of
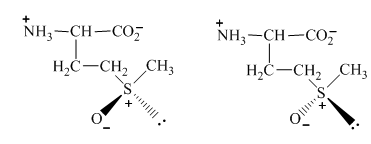
Figure 11
The formation of two separable methionine sulfoxides from the oxidation of
Want to see more full solutions like this?
Chapter 27 Solutions
Organic Chemistry
- this is an organic chemistry question please answer accordindly!! please post the solution draw the figures and post, answer the question in a very simple and straight forward manner thanks!!!!! please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures or diagrams, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this kindly solve all parts and draw it not just word explanations!!arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Select to Edit Arrows H H Select to Add Arrows > H CFCI: Select to Edit Arrows H Select to Edit Arrowsarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





