
Draw the products formed when
a.
b.
c.
d. excess
e.
f.
g.
h.
i. Part (b), then
j.
(a)
Interpretation: The product formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The product formed by the treatment of
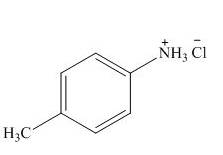
Explanation of Solution
The product formed by the treatment of
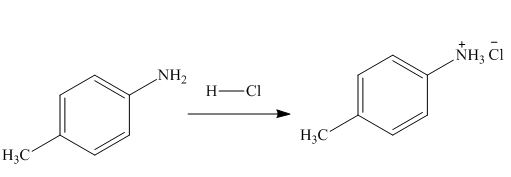
Figure 1
In the above reaction, hydrochloric acid attacks on nitrogen atom of
The product formed by the treatment of
(b)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The products formed by the treatment of
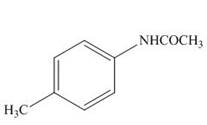
Explanation of Solution
The products formed by the treatment of
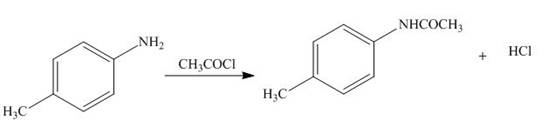
Figure 2
The above reaction indicates that
The products formed by the treatment of
(c)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The products formed by the treatment of
Explanation of Solution
The products formed by the treatment of
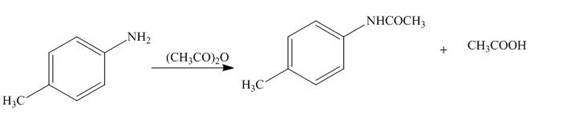
Figure 3
The above reaction implies that
The products formed by the treatment of
(d)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The products formed by the treatment of
Explanation of Solution
The products formed by the treatment of
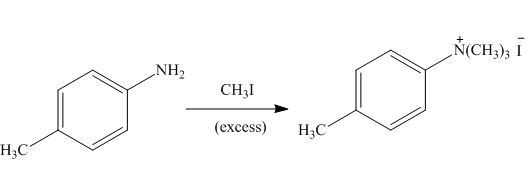
Figure 4
The above reaction indicates that excess of methyl iodide reacts with
The products formed by the treatment of
(e)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The products formed by the treatment of
Explanation of Solution
The products formed by the treatment of
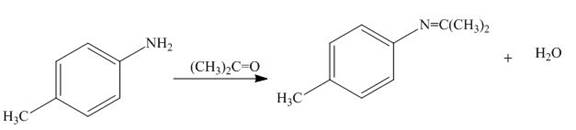
Figure 5
Water gets removed when
The products formed by the treatment of
(f)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The products formed by the treatment of
Explanation of Solution
The products formed by the treatment of
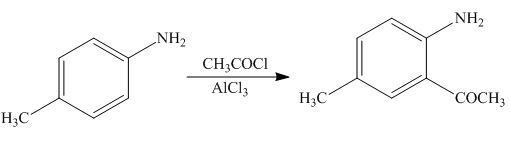
Figure 6
The above reaction indicates that
(g)
Interpretation: The product formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The product formed by the treatment of
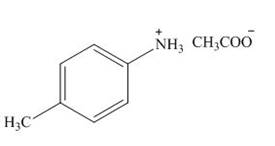
Explanation of Solution
The product formed by the treatment of
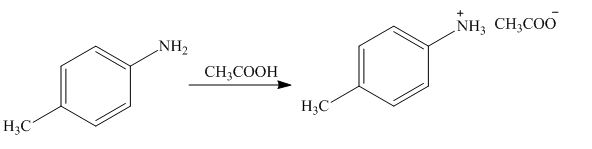
Figure 7
The above reaction indicates that
The product formed by the treatment of
(h)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The product formed by the treatment of
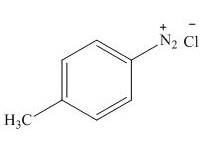
Explanation of Solution
The product formed by the treatment of
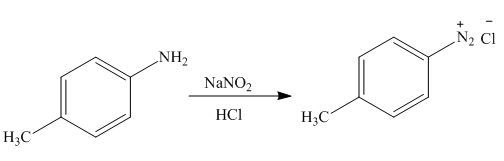
Figure 8
In the above reaction
The product formed by the treatment of
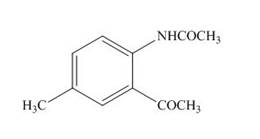
(i)
Interpretation: The product formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The product formed by the treatment of
Explanation of Solution
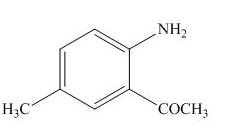
The product formed by the treatment of
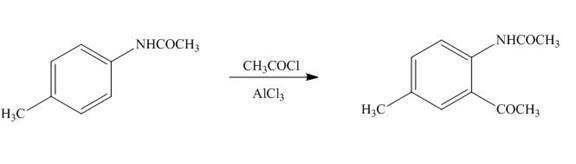
Figure 9
The product formed in part (b) is used as a reactant in the above reaction. It again reacts with
The product formed by the treatment of
(j)
Interpretation: The product formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The product formed by the treatment of
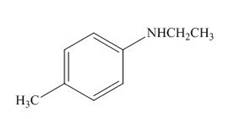
Explanation of Solution
The product formed by the treatment of
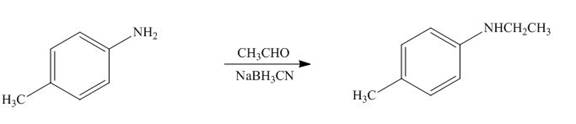
Figure 10
In the above reaction,
The product formed by the treatment of
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry
- MCQ 3: The carbonyl group in aldehyde is A. C=O B. C-O C. CO D. CHOarrow_forward3arrow_forwardDraw the products formed when p-methylaniline (p-CH3C6H4NH2) is treated with each reagent. a. HCl b. CH3COCl c. (CH3CO)2O d. excess CH3I e. (CH3)2C = O f. CH3COCl, AlCl3 g. CH3CO2H h. NaNO2, HCl i. Part (b), then CH3COCl, AlCl j. CH3CHO, NaBH3CNarrow_forward
- Identify each compound as an ether, hemiacetal, or acetal.arrow_forwardWhat products are formed when each alcohol is oxidized with K 2Cr 2O 7? In some cases, no reaction occurs.arrow_forwardDoes the equilibrium favor the reactants or products in each substitution reaction? a. CH;CH2-NH2 Br CH;CH2-Br + "NH2 b. "CN CN + I-arrow_forward
- Classify each alkyl halide as 1°, 2°, or 3°. CH3 c. CHg-C-CHCH3 ČH3 ČI CH;CH2CH,CH,CH2-Br b. d. a.arrow_forwardWhich reagent(s) convert a carbonyl group (C=O) into a methylene group (CH2)? O A. Zn / HCI O B. LIAIH4 / Et20 O C. NABH4 / MeOH D. Na in liq. NH3arrow_forwardWhat products are formed when the following compounds react with ozone and then with dimethyl sulfide?arrow_forward
- Convert propan-2-ol [(CH3)2CHOH] to each compound. You may use any other organic or inorganic compounds.arrow_forwardGive the IUPAC name for each compound (D, E, F)arrow_forwardWhat alkenes are formed when each alcohol is treated with H 2SO 4? Use the Zaitsev rule to predict the major product.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





