
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 25, Problem 25.46P
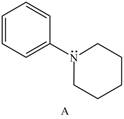
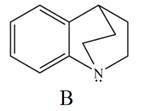
Explain the observed difference in the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The antimalaria properties of quinine (C20H24N202) saved thousands of lives
during construction of the Panama Canal. This substance is a classic example of
the medicinal wealth that tropical forests hold. Both N atoms are basic, and the
N of the amine group is far more basic (pKb=4.94) than the N within the
aromatic ring system (pKb=10.54). A saturated solution of quinine in water is
only 4.55x10-3M. What is the pH of this solution? Record your answer with 2
decimals.
The ammonium ion (pKa = 9.25) has a lower pKa than the methyl ammonium ion (pKa = 10.66). Explain which of the two species is the stronger base
hydrogens on a carbon adjacent to a carbonyl group are far
more acidic than those not adjacent to a carbonyl group. The anion derived from acetone,
for example, is more stable than is the anion derived from ethane. Account for the greater
stability of the anion from acetone.
CH,ČCH, H
CH,CH, H
Acetone
Ethane
pK, 20.2
pK, 51
Chapter 25 Solutions
Organic Chemistry
Ch. 25 - Prob. 25.1PCh. 25 - Prob. 25.2PCh. 25 - Prob. 25.3PCh. 25 - Prob. 25.4PCh. 25 - Prob. 25.5PCh. 25 - Prob. 25.6PCh. 25 - Problem 25.7
Draw the product of each...Ch. 25 - Prob. 25.8PCh. 25 - Prob. 25.9PCh. 25 - Prob. 25.10P
Ch. 25 - Prob. 25.11PCh. 25 - Prob. 25.12PCh. 25 - Prob. 25.13PCh. 25 - Prob. 25.14PCh. 25 - Prob. 25.15PCh. 25 - Prob. 25.16PCh. 25 - Prob. 25.17PCh. 25 - Problem 25.18
Write out steps to show how each of...Ch. 25 - Prob. 25.19PCh. 25 - Prob. 25.20PCh. 25 - Prob. 25.21PCh. 25 - Problem 25.22
Which nitrogen atom in each compound...Ch. 25 - Prob. 25.23PCh. 25 - Prob. 25.24PCh. 25 - Prob. 25.25PCh. 25 - Prob. 25.26PCh. 25 - Prob. 25.27PCh. 25 - Problem 25.28
Draw the major product formed in...Ch. 25 - Prob. 25.29PCh. 25 - Prob. 25.30PCh. 25 - Problem 25.31
Devise a synthesis of each compound...Ch. 25 - Prob. 25.32PCh. 25 - Problem 25.33
What starting materials are needed...Ch. 25 - Problem 25.34
(a) What two components are needed...Ch. 25 - Prob. 25.35PCh. 25 - Prob. 25.36PCh. 25 - 25.37 Varenicline (trade name Chantix) is a drug...Ch. 25 - Give a systematic or common name for each...Ch. 25 - Prob. 25.39PCh. 25 - 25.40 How many stereogenic centers are present in...Ch. 25 - 25.41 Rank the compounds in each group in order of...Ch. 25 - 25.42 Decide which atom in each molecule is most...Ch. 25 - 25.43 Explain why pyrimidine is less basic than...Ch. 25 - 25.44 Rank the nitrogen atoms in each compound in...Ch. 25 - 25.45 Explain why nitroaniline is a stronger base...Ch. 25 - 25.46 Explain the observed difference in the...Ch. 25 - 25.47 Why is pyrrole more acidic than...Ch. 25 - Prob. 25.48PCh. 25 - Prob. 25.49PCh. 25 - Prob. 25.50PCh. 25 - 25.51 How would you separate toluene , benzoic...Ch. 25 - 25.52 Draw the products formed when methylaniline ...Ch. 25 - Prob. 25.53PCh. 25 - Prob. 25.54PCh. 25 - 25.55 Draw the organic products formed in each...Ch. 25 - Prob. 25.56PCh. 25 - 25.57 Identify A, B, and C, three intermediates in...Ch. 25 - Prob. 25.58PCh. 25 - Prob. 25.59PCh. 25 - 25.60 A chiral amine A having the configuration...Ch. 25 - 25.61 Draw a stepwise mechanism for each...Ch. 25 - 25.62 Draw a stepwise mechanism for the following...Ch. 25 - Prob. 25.63PCh. 25 - 25.64 Tertiary aromatic amines react with and ...Ch. 25 - 25.65 Devise a synthesis of each compound from...Ch. 25 - Prob. 25.66PCh. 25 - Prob. 25.67PCh. 25 - Prob. 25.68PCh. 25 - Prob. 25.69PCh. 25 - 25.70 Devise a synthesis of each biologically...Ch. 25 - 25.71 Devise a synthesis of each compound from...Ch. 25 - 25.72 Three isomeric compounds A, B, and C, all...Ch. 25 - 25.73 Treatment of compound D with LiAlH4 followed...Ch. 25 - Prob. 25.74PCh. 25 - 25.75 Rank the following compounds in order of...Ch. 25 - Prob. 25.76PCh. 25 - Prob. 25.77PCh. 25 - Prob. 25.78P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Will acetylene react with sodium hydride according to the following equation to form a salt and hydrogen, H2? Using pKa values given in Table 4.1, calculate Keq for this equilibrium.arrow_forwardAcid-Base Equilibria Many factors contribute to the acidity of organic compounds. Electronegativity, resonance, induction, hybridization, aromaticity, and atomic size, all play a role. In the following comparisons, you are asked to identify the factor(s) that would be most important to analyze when predicting relative acidity, and then to predict the trend in acidity and pKa values. For each of the following pairs of compounds answer the following two multiple-choice questions. 1. What factor(s) are the most important to consider when predicting the relative acidity of the two compounds? a. Electronegativity of the atom possessing the hydrogen. b. Resonance stabilization of the anionic conjugate base. c. Inductive stabilization of the anionic conjugate base. d. Hybridization of the atom possessing the hydrogen. e. The atomic size of the atom possessing the hydrogen.arrow_forwardLow-molecular-weight dicarboxylic acids normally exhibit two different pKa values. Ionization of the first carboxyl group is easier than the second. This effect diminishes with molecular size, and for adipic acid and longer chain dicarboxylic acids, the two acid ionization constants differ by about one pK unit. Why do the two pKa values differ more for the shorter chain dicarboxylic acids than for the longer chain dicarboxylic acids?arrow_forward
- What is the relative trend in acidity and pKa of the two compounds? a. Structure I is the most acidic, and Structure I has the highest pKa. b. Structure I is the most acidic, and Structure I has the lowest pKa. c. Structure II is the most acidic, and Structure I has the highest pKa. d. Structure II is the most acidic, and Structure I has the lowest pKa.arrow_forwardWhich has the larger numerical value? (a) The pKa of a strong acid or the pKa of a weak acid (b) The Ka of a strong acid or the Ka of a weak acidarrow_forwardIn each pair, select the stronger acid. (a) Pyruvic acid (pKa 2.49) or lactic acid (pKa 3.08) (b) Citric acid (pKa1 3.08) or phosphoric acid (pKa1 2.10)arrow_forward
- 11) Which compound is the strongest acid? a) b) □ d) same acidityarrow_forwardUsing pKa Values to Determine Relative Acidity and Basicity Rank the following compounds in order of increasing acidity, and then rank their conjugate bases in order of increasing basicity.arrow_forwardThe following pKa values have been measured. Explain why a hydroxyl group in the para position decreases the acidity while a hydroxyl group in the meta position increases the acidity.arrow_forward
- 16-26 The p/fb of amphetamine is approximately 3.2 Amphetamine (a) Which form of amphetamine (the free base or its conjugate acid) would you expect to be present at pH 1.0, the pH of stomach acid? (b ) Which form of amphetamine would you expect to be present at pH 7.40, the pH of blood plasma?arrow_forwardWrite an equation for the acid-base reaction between 2,4-pentanedione and sodium ethoxide and calculate its equilibrium constant, Keq. The pKa of 2,4-pentanedione is 9; that of ethanol is 15.9.arrow_forward18-28 Arrange these compounds in order of increasing acidity: benzoic acid, benzyl alcohol, phenol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY