
(a)
Interpretation: The more highly oxidized compound is to be identified.
Concept Introduction: In the process of oxidation, oxygen or any other electronegative element is added, and hydrogen or another electropositive element is removed. The process of an atom or ion losing one or more electrons is known as oxidation, according to the electronic notion.
(a)
Answer to Problem 110A
Ethanal is more highly oxidized compound.
Explanation of Solution
The compound that is highly oxidized can be detected with the help of its structure.
The compound that has a low number of hydrogen atoms has the tendency to get more oxidized.
The structure of ethanol and ethanal is given below:
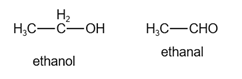
In ethanol, there are six hydrogen atoms.
In ethanal, there are four hydrogen atoms.
Since ethanal has few number of hydrogen atoms, it is highly oxidized.
(b)
Interpretation: The more highly oxidized compound is to be identified.
Concept Introduction: In the process of oxidation, oxygen or any other electronegative element is added, and hydrogen or another electropositive element is removed. The process of an atom or ion losing one or more electrons is known as oxidation, according to the electronic notion.
(b)
Answer to Problem 110A
Ethene is more highly oxidized compound.
Explanation of Solution
The compound that is highly oxidized can be detected with the help of its structure.
The compound that has a low number of hydrogen atoms has the tendency to get more oxidized.
The structure of ethane and ethene is given below:
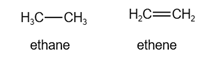
In ethane, there are six hydrogen atoms.
In ethene, there are four hydrogen atoms.
Since ethene has few hydrogen atoms, it is highly oxidized.
(c)
Interpretation: The more highly oxidized compound is to be identified.
Concept Introduction: In the process of oxidation, oxygen or any other electronegative element is added, and hydrogen or another electropositive element is removed. The process of an atom or ion losing one or more electrons is known as oxidation, according to the electronic notion.
(c)
Answer to Problem 110A
Ethanoic acid is a more highly oxidized compound.
Explanation of Solution
The compound that is highly oxidized can be detected with the help of its structure.
The compound that has a low number of hydrogen atoms has the tendency to get more oxidized.
The structure of ethanoic acid and ethanal is given below:
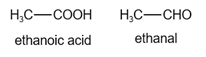
In ethanoic acid, there are four hydrogen atoms.
In ethanal, there are four hydrogen atoms.
Since
(d)
Interpretation: The more highly oxidized compound is to be identified.
Concept Introduction: In the process of oxidation, oxygen or any other electronegative element is added, and hydrogen or another electropositive element is removed. The process of an atom or ion losing one or more electrons is known as oxidation, according to the electronic notion.
(d)
Answer to Problem 110A
Ethyne is more highly oxidized compound.
Explanation of Solution
The compound that is highly oxidized can be detected with the help of its structure.
The compound that has a low number of hydrogen atoms has the tendency to get more oxidized.
The structure of ethyne and ethane is given below:
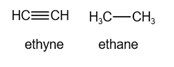
In ethyne, there are two hydrogen atoms.
In ethane, there are six hydrogen atoms.
Since ethyne has a few number of hydrogen atoms, it is highly oxidized.
Chapter 25 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





