
Concept explainers
(a)
Interpretation:
The reagent and condition has to be proposed for step 1,2,3 and 5.
Concept introduction:
Hydrogenolysis:
Nitration: The formation of nitro group in a
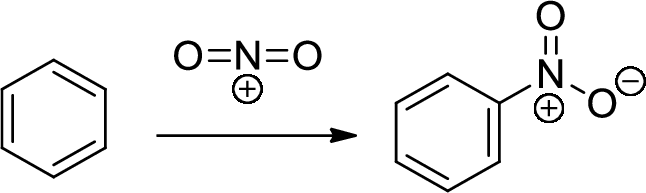
(b)
Interpretation:
The mechanism is to be proposed for iodination of 3-aminobenzoic acid.
Concept introduction:
Electrophilic
Halogenations on benzene:
Halogenation is one of the electrophilic substitution reactions. Halogens reaction with benzene (or electron donating group present in the benzene ring) which gives the corresponding halogenated compound.
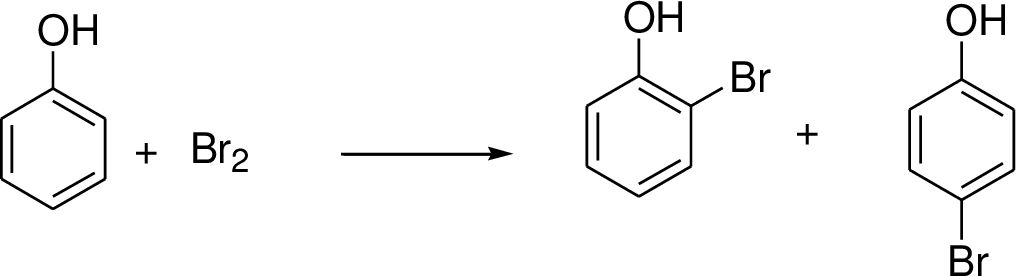
Trending nowThis is a popular solution!

Chapter 23 Solutions
Organic Chemistry, Loose-leaf Version
- Several diamines are building blocks for the synthesis of pharmaceuticals and agro-chemicals. Show how both 1,3-propanediamine and 1,4-butanediamine can be prepared from acrylonitrile.arrow_forwardWhy do you wash the dichloromethane solution of your reductive amination product with sodium bicarbonate, rather than dilute aqueous HCl? a) Sodium bicarbonate is a good method of removing aldehydes from organic solvent.b) The amine product will be protonated by acid and remain in the aqueous layer as a salt.c) Sodium bicarbonate transfers the amine starting material into the aqueous layer.d) Sodium bicarbonate reacts with leftover NaBH(OAc)3 and removes it from the mixture.arrow_forwardWhat are the safety hazards of these chemicals Acetic anhydride (108-24-7) Formic acid (64-18-60) Benzotriazole (95-14-7) N-Formylbenzotriazole (72773-04-7)arrow_forward
- Aspirin is the common name for the compound acetylsalicylic acid, widely used as a fever reducer and as a pain killer. Salicylic acid, whose name comes from Salix, the willow family of plants, was derived from willow bark extracts. In folk medicine, willow bark teas were used as headache remedies and other tonics. Nowadays, salicylic acid is administered in the form of aspirin which is less irritating to the stomach than salicylic acid. To prepare aspirin, salicylic acid is reacted with an excess of acetic anhydride. A small amount of a strong acid is used as a catalyst which speeds up the reaction. In this experiment, phosphoric acid will be used as the catalyst. The excess acetic acid will be quenched with the addition of water. The aspirin product is not very soluble in water so the aspirin product will precipitate when water is added. The synthesis reaction of aspirin is shown below: Actic anhydride 5 ml. Acetic acid Salicylic acid 28 Acetylsalicylie acid Procedure 1) Mix salicylic…arrow_forwardThe reaction of N−bromosuccinimide with 4−methyl−3−nitroanisole has been reported in the chemical literature. This reaction yields a single product in 95% yield. Identify the product formed from this starting material.arrow_forwardIn an attempt to synthesize compound C through a two-step process, a chemist discovered after completing the first step that they had inadvertently produced two distinct compounds, A and B. Upon examining the infrared spectroscopy (IR) results, it was observed that both A and B exhibited peaks indicative of a ketone and an ester group. Please provide the molecular structures of A and B. OEt NaOEt ΕΙΟ A B In a chemical experiment, they noticed that both components, A and B, from a combined sample turned into a new compound, C, during the following stage. The task is to determine what compound C looks like and explain how compound A or B changes into compound C through a reaction. Compound C should be the primary molecule containing carbon created in this process, not just a by-product. A B H3O+, H₂O, A Mechanism = сarrow_forward
- 5 The compounds labeled benzophenone-3 (CH,O,) and benzophenone-5 (CHNAO,S) are found in certain sunscreens. Would you expect a sunscreen made with benzophenone-3 or benzophenone-5 to be more waterproof? Explain your choice.arrow_forwardProvide the systematic name for each of the following isomeric amides with the chemical formula C6H₁1 NO. (Be sure to indicate double bond stereochemistry using (E)/(Z) notation. Indicate stereochemistry in rings with the terms cis or trans. It is not necessary to use italics in writing compound names.) ball & stick + labels N-methylpent-4-enamide An error has been detected in your answer. Check for typos, miscalculations etc. before submitting your answer. ball & stick - + labels N, 3-dimethylbut-3-enamide ball & stick + labels (2Z)-2-methylpent-2-enamidearrow_forwardBriefly describe the role of this molecule as it relates to bees. Propose Two reasonable syntheses of this molecule from benzene. there are multiple syntheses possible (the first proposed synthesis required twelve steps) HO. OHarrow_forward
- How could you differentiate between hexan-3-ol and hexanal using a simple chemical test?arrow_forwardThe NH bobds in ammonia have an IR frequency of 3335 cm^-1, while the PH bonds in phosphine have an IR frequency of 2327 cm^-1. In a single sentence, describe the reason for the difference.arrow_forwardWhich of the following compounds precipitates during a positive test for SN1 reactivity of an alkyl halide? A positive test does not give a precipitate. alcohol complexed to Ag AgBr or AgCl Ag (H₂O) elemental mercury elemental silver alkyl nitratesarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole




