
Organic Chemistry, Loose-leaf Version
8th Edition
ISBN: 9781305865549
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 23, Problem 23.52P
Interpretation Introduction
Interpretation:
The synthesis of N-methylmorpholine has to be shown.
Concept introduction:
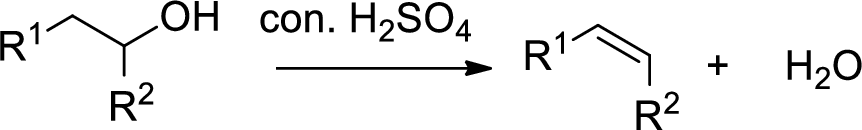
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Define the simplest method to synthesize an amine ?
The following three derivatives of succinimide are anticonvulsants and have found use in the treatment of epilepsy, particularly petit mal seizures.
Ph
Ph
`N'
`N'
ČH3
ČH3
Methsuximide
Ethosuximide
Phensuximide
Following is a synthesis of phensuximide.
CN
Ph
CN
Ph
CN
1. NaOH, H2O
2. HC, Н20
NaOEt
KCN
Ph-CHO
cOOEt
H
cOOEt
NC
COOEt
3. Нeat
Ethyl
cyanoacetate
(A)
(B)
Benzaldehyde
Ph
Ph
Ph
CH;NH2
НООС
СООН
Et0oC
COOEt
`N'
(C)
(D)
ČH3
Phensuximide
Methsuximide is formed by a similar pathway to that shown for phensuximide. Draw the structure of the compound that reacts with ethyl cyanoacetate in the synthesis of
methsuximide.
+
Amines can be prepared by converting other functional groups. Classify these
reactions and choose the correct reagents.
NO₂
NH₂
Three reactions that can be used to prepare amines are shown below.
NH₂
NH₂
NH₂
Classify these reactions.
A) Elimination
B) Substitution
C) Reduction
D) Oxidation
E) Proton transfer reactions
Chapter 23 Solutions
Organic Chemistry, Loose-leaf Version
Ch. 23.1 - Prob. 23.1PCh. 23.2 - Prob. 23.2PCh. 23.2 - Prob. 23.3PCh. 23.2 - Prob. 23.4PCh. 23.5 - Prob. 23.5PCh. 23.5 - Prob. AQCh. 23.5 - What is the hybridization of the nitrogen in...Ch. 23.5 - Prob. CQCh. 23.5 - The pKas of the conjugate acids of aniline and...Ch. 23.5 - Prob. EQ
Ch. 23.5 - Prob. FQCh. 23.5 - Prob. GQCh. 23.5 - Select the stronger acid from each pair of...Ch. 23.6 - Prob. 23.7PCh. 23.6 - Prob. 23.8PCh. 23.6 - Prob. 23.9PCh. 23.7 - Prob. 23.10PCh. 23.8 - Prob. 23.11PCh. 23.8 - Prob. 23.12PCh. 23.8 - Prob. 23.13PCh. 23.9 - Prob. 23.14PCh. 23.10 - In Example 23.15, you considered the product of...Ch. 23 - Prob. 23.16PCh. 23 - Prob. 23.17PCh. 23 - Prob. 23.18PCh. 23 - Prob. 23.19PCh. 23 - Prob. 23.20PCh. 23 - Prob. 23.21PCh. 23 - Prob. 23.22PCh. 23 - Account for the formation of the base peaks in...Ch. 23 - Prob. 23.24PCh. 23 - Select the stronger base from each pair of...Ch. 23 - The pKa, of the conjugate acid of morpholine is...Ch. 23 - Which of the two nitrogens in pyridoxamine (a form...Ch. 23 - Prob. 23.28PCh. 23 - Prob. 23.29PCh. 23 - Prob. 23.30PCh. 23 - Prob. 23.31PCh. 23 - Suppose you have a mixture of these three...Ch. 23 - Prob. 23.33PCh. 23 - Prob. 23.34PCh. 23 - Prob. 23.35PCh. 23 - Prob. 23.36PCh. 23 - Prob. 23.37PCh. 23 - (S)-Glutamic acid is one of the 20 amino acid...Ch. 23 - Prob. 23.39PCh. 23 - Propose a structural formula for the compound...Ch. 23 - Prob. 23.41PCh. 23 - The pyrolysis of acetic esters to give an alkene...Ch. 23 - Propose steps for the following conversions using...Ch. 23 - Show how to bring about each step in this...Ch. 23 - Show how to bring about each step in the following...Ch. 23 - Prob. 23.48PCh. 23 - Prob. 23.49PCh. 23 - Methylparaben is used as a preservative in foods,...Ch. 23 - Prob. 23.51PCh. 23 - Prob. 23.52PCh. 23 - Propose a synthesis for the systemic agricultural...Ch. 23 - Prob. 23.54PCh. 23 - Several diamines are building blocks for the...Ch. 23 - Prob. 23.56PCh. 23 - Prob. 23.57PCh. 23 - Prob. 23.58PCh. 23 - Prob. 23.59PCh. 23 - Following is a retrosynthesis for the coronary...Ch. 23 - Prob. 23.61PCh. 23 - Prob. 23.62PCh. 23 - Given this retrosynthetic analysis, propose a...Ch. 23 - Prob. 23.64PCh. 23 - Following is a series of anorexics (appetite...Ch. 23 - Prob. 23.66PCh. 23 - Prob. 23.67PCh. 23 - Show how the synthetic scheme developed in Problem...Ch. 23 - Prob. 23.69PCh. 23 - Prob. 23.70PCh. 23 - Prob. 23.71PCh. 23 - Prob. 23.72P
Knowledge Booster
Similar questions
- Indicate whether the following statement is true or false. Aliphatic amines are more basic than ammonia, whereas aromatic amines are less basic than ammonia. When amines are reacted with bases, they form ammonium salts. Benzenesulfonyl chloride or p-toluenesulfonylchloride give N-substituted sulfonamides with primary and secondary amines. Derivative of primary amines is insoluble in dilute NaOHarrow_forwardIn the mid-1930s a substance was isolated from a fungus that is a parasite of ryes and other grasses. This alkaloid, lysergic acid, has been of great interest to chemists because of its strange, dramatic action on the human mind. Many derivatives of lysergic acid are known, some with medicinal applications. Perhaps the best known derivative of lysergic acid is the potent hallucinogen lysergic acid diethylamide (LSD): మగవా జి N-H LSD (CH25N;O) Like other alkaloids, LSD is a weak base, with Kp = 7.6 × 107. What is the pH of a 0.94 M solution of LSD? pH =arrow_forward16-26 The p/fb of amphetamine is approximately 3.2 Amphetamine (a) Which form of amphetamine (the free base or its conjugate acid) would you expect to be present at pH 1.0, the pH of stomach acid? (b ) Which form of amphetamine would you expect to be present at pH 7.40, the pH of blood plasma?arrow_forward
- Why is methyl salicylate so easily absorbed through the skin?arrow_forward1 2 3 4 Draw The Structures? Z-Butenedioic acid (maleic acid) 2,5-Dimethylbenzoic acid Z-2-Chloro-3-phenyl-2- propenoic acid (cis-B- chloroallocinnamic acid) Decanedioic acid (sebacic acid) E-3-Phenyl-2-propenoic acid (trans-cinnamic acid)arrow_forwardWhat steps would be used to synthesize an organic amine? Group of answer choices Ketone + oxidizing agent →carboxylic acid + ammonia →organic amine Alkene + acid → alcohol + ammonia →organic amine Ether + acid →ester + ammonia →organic amine Alkane + acid → alcohol + ammonia →organic amine Carboxylic acid + alcohol →ester + ammonia →organic amine Aldehyde + oxidizing agent →carboxylic acid + ammonia → organic aminearrow_forward
- a) What are the difficulties with the following reaction to synthesize an amine. + HBr Br + NH3 NH2 b) Show a possible alternatives to this synthesis.arrow_forwardStanozolol is an anabolic steroid that promotes muscle growth. Althoughstanozolol has been used by athletes and body builders, many physicaland psychological problems result from prolonged use and it is bannedin competitive sports. Explain why the pKa of the N—H bond in the pyrazole ring iscomparable to the pKa of the O—H bond, making it considerably moreacidic than amines such as CH3NH2 (pKa = 40).arrow_forwardFollowing are structural formulas for amphetamine and methamphetamine. H NH, CH3 (a) (b) Amphetamine (racemic) Methamphetamine (racemic) The major central nervous system effects of amphetamine and amphetamine-like drugs are locomotor stimulation, euphoria and excitement, stereotyped behavior, and anorexia. Show how each drug can be synthesized by reductive amination of an ap- propriate aldehyde or ketone and amine.arrow_forward
- LSD (a hallucinogen) and codeine (a narcotic) are structurally more complex derivatives of 2-phenylethanamine. Identify the atoms of 2-phenylethanamine in each of the following compounds.arrow_forwardClassify each amine as a primary, secondary, or tertiary.arrow_forwardIn the 1880's, Acetanilide, sold under the name Antifebrin, was widely used as a pain reliever and fever reducer. However, it had many adverse side effects, including cyanosis as a result of methemoglobinemia. The toxic side effects were the result of a small portion of acetanilide being hydrolyzed to aniline. Acetanilide was discontinued and replaced with phenacetin. Later studies show that both acetanilide and phenacetin are metabolized to acetaminophen. This metabolite, which we know as Tylenol, is responsible for the analgesic and antipyretic properties. Part 1: Show a detailed arrow pushing mechanism of the acid catalyzed hydrolysis of acetanilide to aniline Part 2: Propose a synthesis of Acetaminophen from phenol NH NH NH Phenacetin inophen Acetanilide Attach File Browse Local Files Browse Content Collectionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning