
Organic Chemistry, Loose-leaf Version
8th Edition
ISBN: 9781305865549
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23, Problem 23.55P
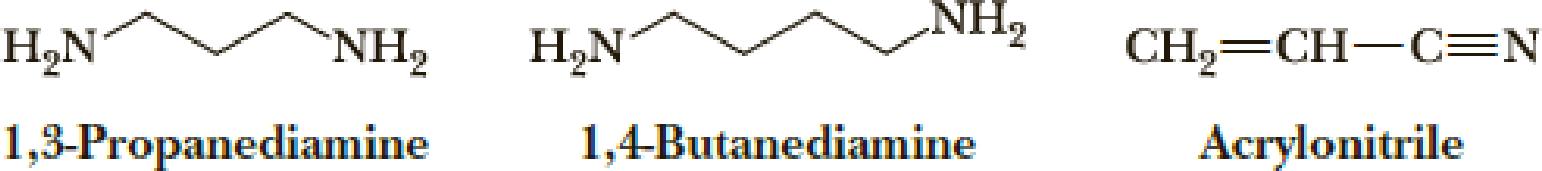
Several diamines are building blocks for the synthesis of pharmaceuticals and agro-chemicals. Show how both 1,3-propanediamine and 1,4-butanediamine can be prepared from acrylonitrile.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Use a series of reactions to show how you can make 2-pentanone and 3-pentanone from 2-pentyne. Name all reactants and intermediate products formed. Indicate the type(s) of reactions required.
Describe the acidity of different carboxylic acids and predict the products obtained when they react with strong bases.
Phenols are aromatic rings with an alcohol functional group attached directly to the ring. These compounds have unique acidity and solubility for alcohol groups. Predict the solubility of this phenol in water.
Chapter 23 Solutions
Organic Chemistry, Loose-leaf Version
Ch. 23.1 - Prob. 23.1PCh. 23.2 - Prob. 23.2PCh. 23.2 - Prob. 23.3PCh. 23.2 - Prob. 23.4PCh. 23.5 - Prob. 23.5PCh. 23.5 - Prob. AQCh. 23.5 - What is the hybridization of the nitrogen in...Ch. 23.5 - Prob. CQCh. 23.5 - The pKas of the conjugate acids of aniline and...Ch. 23.5 - Prob. EQ
Ch. 23.5 - Prob. FQCh. 23.5 - Prob. GQCh. 23.5 - Select the stronger acid from each pair of...Ch. 23.6 - Prob. 23.7PCh. 23.6 - Prob. 23.8PCh. 23.6 - Prob. 23.9PCh. 23.7 - Prob. 23.10PCh. 23.8 - Prob. 23.11PCh. 23.8 - Prob. 23.12PCh. 23.8 - Prob. 23.13PCh. 23.9 - Prob. 23.14PCh. 23.10 - In Example 23.15, you considered the product of...Ch. 23 - Prob. 23.16PCh. 23 - Prob. 23.17PCh. 23 - Prob. 23.18PCh. 23 - Prob. 23.19PCh. 23 - Prob. 23.20PCh. 23 - Prob. 23.21PCh. 23 - Prob. 23.22PCh. 23 - Account for the formation of the base peaks in...Ch. 23 - Prob. 23.24PCh. 23 - Select the stronger base from each pair of...Ch. 23 - The pKa, of the conjugate acid of morpholine is...Ch. 23 - Which of the two nitrogens in pyridoxamine (a form...Ch. 23 - Prob. 23.28PCh. 23 - Prob. 23.29PCh. 23 - Prob. 23.30PCh. 23 - Prob. 23.31PCh. 23 - Suppose you have a mixture of these three...Ch. 23 - Prob. 23.33PCh. 23 - Prob. 23.34PCh. 23 - Prob. 23.35PCh. 23 - Prob. 23.36PCh. 23 - Prob. 23.37PCh. 23 - (S)-Glutamic acid is one of the 20 amino acid...Ch. 23 - Prob. 23.39PCh. 23 - Propose a structural formula for the compound...Ch. 23 - Prob. 23.41PCh. 23 - The pyrolysis of acetic esters to give an alkene...Ch. 23 - Propose steps for the following conversions using...Ch. 23 - Show how to bring about each step in this...Ch. 23 - Show how to bring about each step in the following...Ch. 23 - Prob. 23.48PCh. 23 - Prob. 23.49PCh. 23 - Methylparaben is used as a preservative in foods,...Ch. 23 - Prob. 23.51PCh. 23 - Prob. 23.52PCh. 23 - Propose a synthesis for the systemic agricultural...Ch. 23 - Prob. 23.54PCh. 23 - Several diamines are building blocks for the...Ch. 23 - Prob. 23.56PCh. 23 - Prob. 23.57PCh. 23 - Prob. 23.58PCh. 23 - Prob. 23.59PCh. 23 - Following is a retrosynthesis for the coronary...Ch. 23 - Prob. 23.61PCh. 23 - Prob. 23.62PCh. 23 - Given this retrosynthetic analysis, propose a...Ch. 23 - Prob. 23.64PCh. 23 - Following is a series of anorexics (appetite...Ch. 23 - Prob. 23.66PCh. 23 - Prob. 23.67PCh. 23 - Show how the synthetic scheme developed in Problem...Ch. 23 - Prob. 23.69PCh. 23 - Prob. 23.70PCh. 23 - Prob. 23.71PCh. 23 - Prob. 23.72P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1-Octen-3-ol is a potent mosquito attractant commonly used in mosquito traps. A number of reactions, including hydrogenation, will transform 1-octen-3-ol into a less effective molecule. Draw the structure of a hydrogenation product of 1-octen-3-ol.arrow_forwardWhen the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forwardGive equations for the following reactions and name the products: 6. Reaction of butanoic acid with sodium 7. Reaction of ethanoic acid with potassium hydroxide 8. Reaction of propanoic acid with sodium carbonate 9. Reaction of methanol and methanoic acid in the presence of a sulphuric acid catalyst 10. Reaction of butylamine with hydrochloric acidarrow_forward
- Hydration of aldehydes and ketones can be catalyzed by acid or base. Bases catalyze hydration by: protonating the carbonyl oxygen making the carbonyl group more electrophilic employing hydroxide ion, which is a better nucleophile than water making the carbonyl group less electrophilic shifting the equilibrium position of the reaction to favor productsarrow_forwardEster compounds often have a sweet, pleasant odor. Many characteristic fruit scents are largely due to the natural presence of one or more ester compounds. As such, artificial scents for foods are often composed of complex mixtures of various esters. The exact identity and ratio of ingredients that compose a particular scent are closely guarded secrets in the food and fragrance industry. Suppose that you are a chemist working for a company that i creating a new line of air fresheners. The company is considering three scents: apple, pear, and pineapple. The project manager has asked you to prepare the ester compounds that are largely responsible for these scents. The structural formulas for these ester compounds are shown here: Alcohols for Air Freshener Project Molar mass Density Cost, per (g/mL) Reagent (g/mol) 1.00 L methanol 32.04 0.79 $46.20 ethanol 46.07 0.79 $112.00 1-propanol 60.10 0.80 $72.70 1-butanol 74.12 0.81 $72.60 Use the structural formulas of the alcohols and…arrow_forwardUpon completion of a chemical reaction, you find you have a mixture of benzoic acid and ethyl benzoate. Propose a procedure to separate the ethyl benzoate from the mixture. You should look up the structures of benzoic acid and ethyl benzoate.arrow_forward
- The acid-catalyzed dehydration of 2,3-dimethyl-3-pentanol yields three alkene products. What are the names of the three alkenes? Which of the three alkenes is the major product?arrow_forwardTerpineol has 5 isomers. Write their structures and describe why chemically (optically) they receive those names. Number the carbons for a better description.arrow_forwardGive the reaction equation of an acid catalyzed reaction of 2-methyl-2-butene with methanolarrow_forward
- CH3 CH3 H cyclopentamine The synthesis of cyclopentamine, an amphetamine-like central nervous system stimulant, from materials of five carbons or fewer involves several steps, one of which is shown below. Draw the structure(s) of the major organic product(s) of the following reaction: Mg, dry ether Br productarrow_forwardWhy are carbonyl compounds considered weakly acidic? Would you expect carbonyl compounds to be more acidic than alkanes? Explain.arrow_forwardAcetylene reacts with sodium amide in the presence of propyl halide produces aldehyde produces ketones It produces 2-pentanearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License