
Concept explainers
(a)
Interpretation:
Synthesis of benzylamine from the given starting material has to be shown.
Concept Introduction:
Preparation of imine:
An imine is a compound having
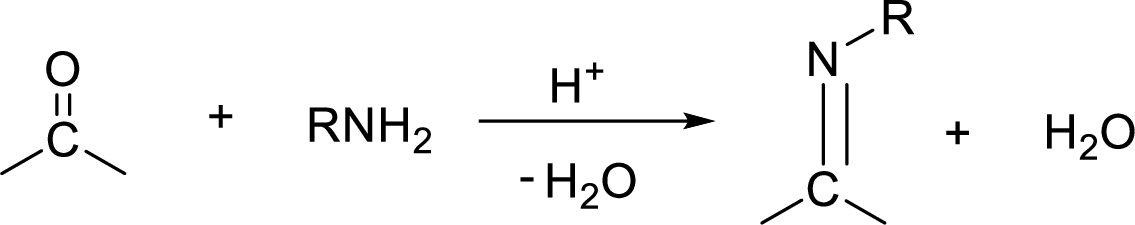
Reduction: If electrons are gained to a species or hydrogen atoms are added to a species or oxygen atom gets removed from a species during a
(b)
Interpretation:
Synthesis of benzylamine from the given starting material has to be shown.
Concept Introduction:
Amide Hydrolysis: In presence of base, amide reacts with water to form the corresponding amine and
(c)
Interpretation:
Synthesis of benzylamine from the given starting material has to be shown.
Concept Introduction:
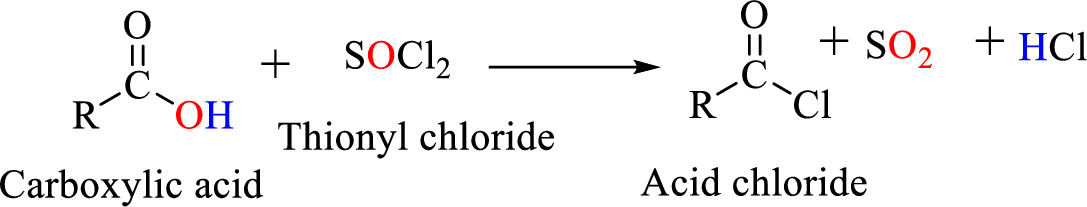
Preparation of amine: A primary amine is formed when an
Thionyl chloride:
(d)
Interpretation:
Synthesis of benzylamine from the given starting material has to be shown.
Concept Introduction:
Thionyl chloride:
(e)
Interpretation:
Synthesis of benzylamine from the given starting material has to be shown.
Concept Introduction:
Acid chlorides are most often prepared by treating a carboxylic acid with thionyl chloride.
Amide Formation: Amide is formed when an acid chloride reacts with an amine or ammonia.
Here, the chlorine atom that is attached to the carbonyl carbon atom of the acid chloride is being replaced by
Reduction: If electrons are gained to a species or hydrogen atoms are added to a species or oxygen atom gets removed from a species during a chemical reaction is known as reduction. In a reaction,
(f)
Interpretation:
Synthesis of benzylamine from the given starting material has to be shown.
Concept Introduction:
Preparation of amide: An amide is formed when an ester is reacted with ammonia.
Reduction: If electrons are gained to a species or hydrogen atoms are added to a species or oxygen atom gets removed from a species during a chemical reaction is known as reduction. In a reaction,
Trending nowThis is a popular solution!

Chapter 23 Solutions
Organic Chemistry, Loose-leaf Version
- b) The Wolf-Kishner reduction is a reaction used in Organic Chemistry to convert carbonyl functionalities into methylene group. The reaction was used to convert an aldehyde or ketone to an alkane using hydrazine, base and thermal conditions. The mechanism begins with the attack of hydrazine of the aldehyde or ketone. Stage 1: The reaction of aldehyde/ketone with hydrazine to produce hydrazine Stage 2: Reaction with the base and heat to convert hydrozone to alkane Write the mechanism of the reaction.arrow_forwardMatch each reagent to the product that it forms. Multiple reagents may form the same product. HO Reagent Reagents SOCI2, pyridine: C CISO2CH3, pyridine: E HCI: A PCI 3: A A) B) "It eft ft D) E) F) "got soft soft MSO MsO CIarrow_forwardShow how to synthesize 3-nitro-4-propoxybenzoic acid from phenol, C6H5OH and any reagents that you need.arrow_forward
- W X Select reagent W and Y 1 HNO3 H₂SO4 2 CH3C1₂ AICI 3 3 Cl₂, FeCl3 ) 4 503, H₂SO4 NO₂ S0₂tarrow_forwardPropose a mechanism for the reaction of benzyl acetate with methylamine. Label theattacking nucleophile and the leaving group, and draw the transition state in which theleaving group leaves.arrow_forwardStarting with cyclohexanone and ethanol as the only organic reagents, use any inorganic reagents to propose a synthesis for the target molecule.arrow_forward
- Draw the products formed when p-methylaniline (p-CH3C6H4NH2) istreated with following reagent. CH3CHO, NaBH3CNarrow_forwardShow how to convert ethylene to 1,2-Ethanediol compound.arrow_forwardyou are performing an acyl nucleophilic substitution reaction using sodium acetate, acetic anhydride, salicyclic acid, and pyridine. show a mechanism for the reaction, and label the nucleophile, acyl compound, and the catalyst.arrow_forward
- Mechanism of azide synthesis: Step 1: Nucleophilic substitution of alkyl halide with sodium azide to form an alkyl azide. Step 2: Reduction of alkyl azide with a reducing agent such as sodium borohydride or lithium aluminum hydride to form an alkylamine. Mechanism of alkylation of ammonia: Step 1: The alkyl halide undergoes a nucleophilic substitution reaction with ammonia gas to form an intermediate alkylamine. Step 2: The intermediate alkylamine is deprotonated by the catalyst to form the final alkylamine.arrow_forwardSynthesize 2-pentanone from 1-butyne. Indicate all the reagents needed.arrow_forwardSuggest possible synthetic route for following transformations. Show the reagents, intermediates and products.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


