
a.
Interpretation:
The type of organic reaction that converts
Concept introduction:
Structural isomers have same number of atoms of element in the compounds but the position of groups is different. An
a.
Explanation of Solution
Addition with hydrogen gas and platinum results in the reduction of double bond and formation of alkane from alkene takes place.
The general reaction is represented as follows:
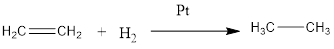
Thus, it is an addition reaction.
b.
Interpretation:
The type of organic reaction that converts alkyl halide to alcohol needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix,
b.
Explanation of Solution
An alkyl halide can be converted to alcohol after addition of water or hydroxide. This is a nucleophillic substitution reaction.
The general reaction is represented as follows:
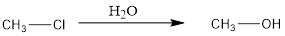
This is an addition reaction. It can also known as hydrolysis reaction as water is added to the alkyl halide. Also, there is substitution of −OH group takes place at the place of Cl thus, it is a type of substitution reaction as well.
c.
Interpretation:
The type of organic reaction that converts alkyl halide to alkene needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
c.
Explanation of Solution
Alkyl halide form alkene after treatment with strong base. The reaction is 1, 2-eliminatio reaction. The bases generally used are NaOH or KOH.
The general reaction is represented as follows:

The above reaction is an elimination reaction.
d.
Interpretation:
The type of organic reaction that converts alcohol to alkyl halide needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
d.
Explanation of Solution
An alcohol can be converted to alkyl halide after reaction with H-X where X is a halide group.
The general reaction is represented as follows:
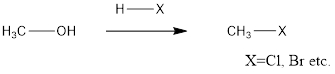
The above reaction is a substitution reaction.
e.
Interpretation:
The type of organic reaction that converts alkyl halide to alkene needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
e.
Explanation of Solution
Alkyl halide form alkene after treatment with strong base. The reaction is 1, 2-eliminatio reaction. The bases generally used are NaOH or KOH.
The general reaction is represented as follows:

This is an elimination reaction.
f.
Interpretation:
The type of organic reaction that converts alkene to alcohol needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
f.
Explanation of Solution
The conversion of alkene to alcohol takes place after reaction with steam at definite temperature and pressure in the presence of phosphoric acid catalyst.
The general reaction is represented as follows:
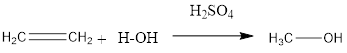
This is an acid catalyzed hydration reaction.
Chapter 22 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Human Anatomy & Physiology (2nd Edition)
Human Physiology: An Integrated Approach (8th Edition)
Organic Chemistry (8th Edition)
Campbell Biology (11th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Campbell Biology in Focus (2nd Edition)
- For the compound: C8H17NO2 Use the following information to come up with a plausible structure: 8 This compound has "carboxylic acid amide" and ether functional groups. The peaks at 1.2ppm are two signals that are overlapping one another. One of the two signals is a doublet that represents 6 hydrogens; the other signal is a quartet that represents 3 hydrogens.arrow_forwardVnk the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest bolling point, choose 2 next to the substance with the next highest boiling point, and so on. substance C D chemical symbol, chemical formula or Lewis structure. CH,-N-CH, CH, H H 10: H C-C-H H H H Cale H 10: H-C-C-N-CH, Bri CH, boiling point (C) Сен (C) B (Choosearrow_forwardPlease help me find the 1/Time, Log [I^-] Log [S2O8^2-], Log(time) on the data table. With calculation steps. And the average for runs 1a-1b. Please help me thanks in advance. Will up vote!arrow_forward
- Q1: Answer the questions for the reaction below: ..!! Br OH a) Predict the product(s) of the reaction. b) Is the substrate optically active? Are the product(s) optically active as a mix? c) Draw the curved arrow mechanism for the reaction. d) What happens to the SN1 reaction rate in each of these instances: 1. Change the substrate to Br "CI 2. Change the substrate to 3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF 4. Increase the substrate concentration by 3-fold.arrow_forwardExperiment 27 hates & Mechanisms of Reations Method I visual Clock Reaction A. Concentration effects on reaction Rates Iodine Run [I] mol/L [S₂082] | Time mo/L (SCC) 0.04 54.7 Log 1/ Time Temp Log [ ] 13,20] (time) / [I] 199 20.06 23.0 30.04 0.04 0.04 80.0 22.8 45 40.02 0.04 79.0 21.6 50.08 0.03 51.0 22.4 60-080-02 95.0 23.4 7 0.08 0-01 1970 23.4 8 0.08 0.04 16.1 22.6arrow_forward(15 pts) Consider the molecule B2H6. Generate a molecular orbital diagram but this time using a different approach that draws on your knowledge and ability to put concepts together. First use VSEPR or some other method to make sure you know the ground state structure of the molecule. Next, generate an MO diagram for BH2. Sketch the highest occupied and lowest unoccupied MOs of the BH2 fragment. These are called frontier orbitals. Now use these frontier orbitals as your basis set for producing LGO's for B2H6. Since the BH2 frontier orbitals become the LGOS, you will have to think about what is in the middle of the molecule and treat its basis as well. Do you arrive at the same qualitative MO diagram as is discussed in the book? Sketch the new highest occupied and lowest unoccupied MOs for the molecule (B2H6).arrow_forward
- Q8: Propose an efficient synthesis of cyclopentene from cyclopentane.arrow_forwardQ7: Use compound A-D, design two different ways to synthesize E. Which way is preferred? Please explain. CH3I ONa NaOCH 3 A B C D E OCH3arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward
- (10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





