
a.
Interpretation:
The structure of 2, 2-dichloro-3-pentanone needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix,
a.
Explanation of Solution
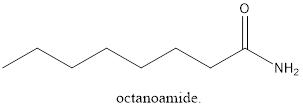
The name of the compound is 2, 2-dichloro-3-pentanone.
From the name, it can be seen that there are 5 carbon atoms in the main carbon chain. Also, there are 2 chlorine groups and 2nd position and
The structure of the carbonyl compound will be:
b.
Interpretation:
The structure of 4-methylpentanal needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
b.
Explanation of Solution
The name of the compound is 4-methylpentanal.
From the name, it can be seen that there are 5 carbon atoms in the main carbon chain. Also, there is an
The structure of compound will be:
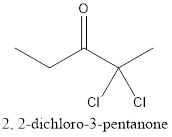
c.
Interpretation:
The structure of isopropyl hexanoate needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
c.
Explanation of Solution
The given compound is isopropyl hexanoate. There is an ester group with isopropyl and hexane group linked together.
The structure of the carbonyl group compound is as follows:
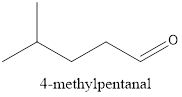
d.
Interpretation:
The structure of octanoamide needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
d.
Explanation of Solution
The given compound is octanoamide.
The carbon chain has 8 carbon atoms and an amide functional group.
The structure of compound will be:
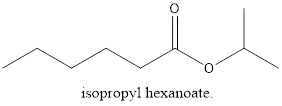
e.
Interpretation:
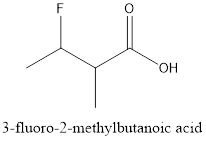
The structure of 3-fluoro-2-methylbutanoic acid needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
e.
Explanation of Solution
The given compound is 3-fluoro-2-methylbutanoic acid. From the name, it has 4 carbon atoms in the chain and a
The structure of compound will be:
f.
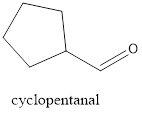
Interpretation:
The structure of cyclopentanal needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
f.
Explanation of Solution
The given compound is cyclopentanal.
From the name there is cyclic ring with 5 carbon atoms. Also, there is an aldehyde group.
The structure of compound will be:
g.
Interpretation:
The structure of hexyl methanoate needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
g.
Explanation of Solution
The given compound is hexyl methanoate. From the name, there is an ester group. Also, there is a hexyl and methane group which is joined together.
Thus, the structure of compound will be:
Chapter 22 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Organic Chemistry (9th Edition)
Introductory Chemistry (6th Edition)
Chemistry: A Molecular Approach
Inorganic Chemistry
Chemistry: A Molecular Approach (4th Edition)
Chemistry: The Central Science (13th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





