
Concept explainers
a. Use bond energies (see Table 3-3) to estimate ΔH for the reaction of two molecules of glycine to form a peptide linkage.
b. Would you predict ΔS to favor the formation of peptide linkages between two molecules of glycine?
c. Would you predict the formation of proteins to be a spontaneous process?
(a)
Interpretation: By using given bond energies the enthalpy
Concept introduction: When two or more amino acids are linked in a chain, a compound called peptide is formed in which the carboxyl group of each acid is joined to the amino group of the next forming a
To determine: The
Answer to Problem 142AE
Answer
The
Explanation of Solution
Explanation
The enthalpy
The reaction between two molecules of glycine to form peptide linkage is,
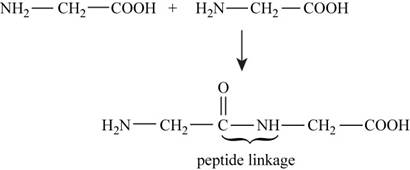
Figure 1
The enthalpy of the reaction is calculated by using the formula,
In the given reaction shown in Figure 1 bonds are broken and formed during peptide formation and energy is absorbed and released. The bond energies are shown in (Table 3-3) respectively.
Therefore, the energy required to break the bonds is calculated as,
Similarly, the energy released when the bonds are formed are calculated as,
Therefore, the total enthalpy for the reaction is calculated by using the formula,
Hence, the total enthalpy of formation for the reaction of two molecules of glycine to form a peptide linkage is positive. It is an endothermic reaction.
(b)
Interpretation: By using given bond energies the enthalpy
Concept introduction: When two or more amino acids are linked in a chain, a compound called peptide is formed in which the carboxyl group of each acid is joined to the amino group of the next forming a
To determine: The entropy
Answer to Problem 142AE
Answer
The entropy
Explanation of Solution
Explanation
The entropy
The reaction between two molecules of glycine to form a peptide linkage is found to be an endothermic process because of the positive value of enthalpy. In the system an endothermic process absorbs heat from the surrounding and therefore, decreases the entropy of the surrounding because the molecular motion in the surrounding decreases. Therefore, for the given reaction
(c)
Interpretation: By using given bond energies the enthalpy
Concept introduction: When two or more amino acids are linked in a chain, a compound called peptide is formed in which the carboxyl group of each acid is joined to the amino group of the next forming a
To determine: The formation of proteins is a spontaneous process or not.
Answer to Problem 142AE
Answer
Formation of proteins is a non-spontaneous process.
Explanation of Solution
Explanation
The formation of proteins is a non-spontaneous process.
The formation of proteins occurs when large numbers of amino acids are joined through peptide linkage. The formation of peptide linkage is a non-spontaneous process as stated above due to the negative value of
Want to see more full solutions like this?
Chapter 22 Solutions
Chemistry
- Indicate the correct answer.a) In boranes, the B-B bonds are the most reactive.b) The B-H-B bonds are the reactive centers in the B2H6 molecule.arrow_forwardIn boranes, the B-B bonds are the most reactive.arrow_forwardThe B-H-B bonds are the reactive centers in the B2H6 molecule. Correct?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





