
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Please help with these drawings! I only have an hour left!
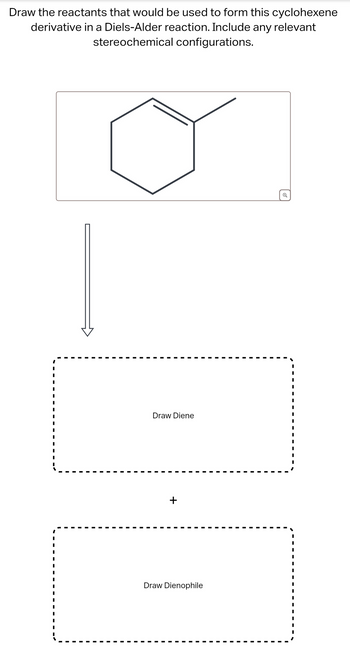
Transcribed Image Text:Draw the reactants that would be used to form this cyclohexene
derivative in a Diels-Alder reaction. Include any relevant
stereochemical configurations.
Draw Diene
+
Draw Dienophile
Q
I
I
I
I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Draw the reactants that would react to form this cyclohexene derivative in a Diels-Alder reaction. Include any relevant stereochemical configurations. { Edit Diene + Draw Dienophile Qarrow_forwardDraw the reactants that would be used to form this cyclohexene derivative in a Diels- Alder reaction. Include any relevant stereochemical configurations. Draw Diene + ·00 aarrow_forwardDraw the reactants that would react to form this cyclohexene derivative in a Diels-Alder reaction. Include any relevant stereochemical configurations. Draw Diene ✔ Iarrow_forward
- Please make your drawings clear! Thank you so mucharrow_forwardA2 What is the inverse electron demand Diels–Alder reaction? Please pick a pair of a diene and a dienophile from the following dienes and dienophile that will undergo this type of reaction. Please show how this reaction works using Frontier Molecular Orbitals. What is the reaction product?arrow_forwardOf the three 1,4-diphenyl-1,3-butadiene isomers (E,E or E,Z or Z,Z) indicate the most suitable diene that can be used as a reactant in a Diels-Alder reaction. Explain your choice.arrow_forward
- Can i get help with this problem?arrow_forwardThis is a Diels-Alder reaction between furan + maleic anhydride (endo and exo products). For each cycloaddition product, draw in all hydrogen atoms, and write the molecular formula below.arrow_forwardCompounds P and Q can undergo a Diels-Alder reaction to form two regioisomeric products R and S as shown in Figure 5. OMe O C8H12O2 R C8H12O2 S Figure 5 Draw the chemical structures of regioisomeric compounds R and S. Using possible resonance contributors of P and Q predict which of the two regioisomers will be favoured in the reaction. Using curly arrows, draw the mechanism for the reaction of P and Q to form the dominant regioisomer you have predicted in your answer to part (ii) above.arrow_forward
- 7. At room temperature cyclopentadiene reacts with itself to form dicyclopentadiene in a Diels-Alder reaction. a) draw the self reaction of cyclopentadiene 20°C. b) When dicyclopentadiene is heated to boiling (170°C), the retor-Diels-Alder reaction occurs producing 2 moles of cyclopentadiene. Explain this observation in terms of free energy, enthalpy and entropy. c) If the AHxn = -75 kJ/mol and the ASpxn = -226 J/mol K, What temperature would be required for the reaction to be at equilibrium (Keq = 1).arrow_forwardDraw the starting diene and dieneophilearrow_forward6. Predict the major product for the following Diels-Alder reaction. 7. Which diene and dienophile would react to give the following Diels-Alder product? Сон 8. Which diene and dienophile would react to give the following Diels-Alder product? Illarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
