
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 51E
Identify each of the following compounds as a
a. Anthraquinone, an important starting material in the manufacture of dyes:
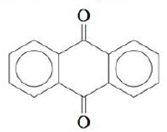
b. 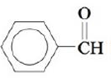
c. 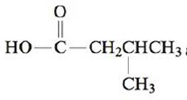
d. 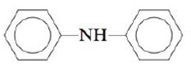
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
My question is whether HI adds to both double bonds, and if it doesn't, why not?
Strain Energy for Alkanes
Interaction / Compound kJ/mol kcal/mol
H: H eclipsing
4.0
1.0
H: CH3 eclipsing
5.8
1.4
CH3 CH3 eclipsing
11.0
2.6
gauche butane
3.8
0.9
cyclopropane
115
27.5
cyclobutane
110
26.3
cyclopentane
26.0
6.2
cycloheptane
26.2
6.3
cyclooctane
40.5
9.7
(Calculate your answer to the nearest 0.1 energy unit, and be sure to specify units, kJ/mol or kcal/mol. The answer is case
sensitive.)
H.
H
Previous
Next
A certain half-reaction has a standard reduction potential Ered +1.26 V. An engineer proposes using this half-reaction at the anode of a galvanic cell that
must provide at least 1.10 V of electrical power. The cell will operate under standard conditions.
Note for advanced students: assume the engineer requires this half-reaction to happen at the anode of the cell.
Is there a minimum standard reduction
potential that the half-reaction used at
the cathode of this cell can have?
If so, check the "yes" box and calculate
the minimum. Round your answer to 2
decimal places. If there is no lower
limit, check the "no" box..
Is there a maximum standard reduction
potential that the half-reaction used at
the cathode of this cell can have?
If so, check the "yes" box and calculate
the maximum. Round your answer to 2
decimal places. If there is no upper
limit, check the "no" box.
yes, there is a minimum.
1
red
Πν
no minimum
Oyes, there is a maximum.
0
E
red
Dv
By using the information in the ALEKS…
Chapter 22 Solutions
Chemistry
Ch. 22 - What is a hydrocarbon? What is the difference...Ch. 22 - Prob. 2RQCh. 22 - What are aromatic hydrocarbons? Benzene exhibits...Ch. 22 - Summarize the nomenclature rules for alkanes,...Ch. 22 - What functional group distinguishes each of the...Ch. 22 - Distinguish between isomerism and resonance....Ch. 22 - Prob. 7RQCh. 22 - Prob. 8RQCh. 22 - Prob. 9RQCh. 22 - Prob. 10RQ
Ch. 22 - Prob. 11RQCh. 22 - Describe the structural differences between DNA...Ch. 22 - Prob. 1QCh. 22 - Prob. 2QCh. 22 - Prob. 3QCh. 22 - Prob. 4QCh. 22 - Prob. 5QCh. 22 - Prob. 6QCh. 22 - Prob. 7QCh. 22 - Give an example reaction that would yield the...Ch. 22 - Prob. 9QCh. 22 - Prob. 10QCh. 22 - Prob. 11QCh. 22 - Prob. 12QCh. 22 - Is the primary, secondary, or tertiary structure...Ch. 22 - Prob. 14QCh. 22 - Prob. 15ECh. 22 - Prob. 16ECh. 22 - Draw all the structural isomers for C8H18 that...Ch. 22 - Draw all the structural isomers for C8H18 that...Ch. 22 - Prob. 19ECh. 22 - Prob. 20ECh. 22 - Draw the structural formula for each of the...Ch. 22 - Prob. 22ECh. 22 - Prob. 23ECh. 22 - Name each of the following cyclic alkanes, and...Ch. 22 - Prob. 25ECh. 22 - Draw the structures for two examples of...Ch. 22 - Name each of the following alkenes. a. CH2 = CH ...Ch. 22 - Name each of the following alkenes or alkynes. a....Ch. 22 - Prob. 29ECh. 22 - Give the structure for each of the following. a....Ch. 22 - Prob. 31ECh. 22 - Cumene is the starting material for the industrial...Ch. 22 - Name each of the following. a. b. CH3CH2CH2CCl3 c....Ch. 22 - Prob. 34ECh. 22 - There is only one compound that is named...Ch. 22 - Prob. 36ECh. 22 - Prob. 37ECh. 22 - Prob. 38ECh. 22 - Draw all the structural isomers of C5H10. Ignore...Ch. 22 - Prob. 40ECh. 22 - Draw all the structural and geometrical (cistrans)...Ch. 22 - Draw all the structural and geometrical (cistrans)...Ch. 22 - Draw all structural and geometrical (cistrans)...Ch. 22 - Prob. 44ECh. 22 - Prob. 45ECh. 22 - Prob. 46ECh. 22 - Prob. 47ECh. 22 - Prob. 48ECh. 22 - If one hydrogen in a hydrocarbon is replaced by a...Ch. 22 - There are three isomers of dichlorobenzene, one of...Ch. 22 - Identify each of the following compounds as a...Ch. 22 - Prob. 52ECh. 22 - Mimosine is a natural product found in large...Ch. 22 - Minoxidil (C9H15N5O) is a compound produced by...Ch. 22 - For each of the following alcohols, give the...Ch. 22 - Prob. 56ECh. 22 - Prob. 58ECh. 22 - Prob. 59ECh. 22 - Prob. 60ECh. 22 - Prob. 61ECh. 22 - Prob. 62ECh. 22 - Prob. 63ECh. 22 - Prob. 64ECh. 22 - Prob. 65ECh. 22 - Prob. 66ECh. 22 - Prob. 67ECh. 22 - Prob. 68ECh. 22 - Prob. 69ECh. 22 - Prob. 70ECh. 22 - Prob. 71ECh. 22 - Prob. 72ECh. 22 - Prob. 73ECh. 22 - Prob. 74ECh. 22 - Prob. 75ECh. 22 - Prob. 76ECh. 22 - Prob. 77ECh. 22 - Prob. 78ECh. 22 - Super glue contains methyl cyanoacrylate, which...Ch. 22 - Prob. 80ECh. 22 - Prob. 81ECh. 22 - Prob. 82ECh. 22 - Prob. 83ECh. 22 - Prob. 84ECh. 22 - Prob. 85ECh. 22 - Prob. 86ECh. 22 - Prob. 87ECh. 22 - Prob. 88ECh. 22 - Prob. 89ECh. 22 - Prob. 90ECh. 22 - Aspartame, the artificial sweetener marketed under...Ch. 22 - Prob. 92ECh. 22 - Prob. 93ECh. 22 - Draw the structures of the tripeptides glyalaser...Ch. 22 - Prob. 95ECh. 22 - Prob. 96ECh. 22 - Prob. 97ECh. 22 - In general terms, what does the tertiary structure...Ch. 22 - Give an example of amino acids that could give...Ch. 22 - What types of interactions can occur between the...Ch. 22 - Prob. 101ECh. 22 - Prob. 102ECh. 22 - Prob. 103ECh. 22 - Prob. 104ECh. 22 - Prob. 105ECh. 22 - Prob. 106ECh. 22 - Prob. 107ECh. 22 - Prob. 108ECh. 22 - Prob. 109ECh. 22 - Prob. 110ECh. 22 - Prob. 111ECh. 22 - Prob. 114ECh. 22 - Prob. 115ECh. 22 - Prob. 116ECh. 22 - Prob. 117ECh. 22 - Prob. 118ECh. 22 - The base sequences in mRNA that code for certain...Ch. 22 - Prob. 120ECh. 22 - Prob. 121AECh. 22 - Prob. 122AECh. 22 - Prob. 123AECh. 22 - Prob. 124AECh. 22 - Prob. 125AECh. 22 - Prob. 126AECh. 22 - Prob. 127AECh. 22 - Prob. 128AECh. 22 - Explain why methyl alcohol is soluble in water in...Ch. 22 - Prob. 130AECh. 22 - Prob. 131AECh. 22 - Prob. 132AECh. 22 - Prob. 133AECh. 22 - Prob. 134AECh. 22 - Prob. 135AECh. 22 - Prob. 136AECh. 22 - Prob. 137AECh. 22 - Prob. 138AECh. 22 - Prob. 139AECh. 22 - Prob. 140AECh. 22 - Prob. 141AECh. 22 - a. Use bond energies (see Table 3-3) to estimate H...Ch. 22 - Prob. 143AECh. 22 - Prob. 144AECh. 22 - All amino acids have at least two functional...Ch. 22 - Prob. 146AECh. 22 - Prob. 147AECh. 22 - In glycine, the carboxylic acid group has Ka = 4.3...Ch. 22 - Name each of the following alkanes. a....Ch. 22 - Prob. 150CWPCh. 22 - Name each of the following cyclic alkanes. a. b....Ch. 22 - Name each of the following alkenes and alkynes. a....Ch. 22 - a. Name each of the following alcohols. b. Name...Ch. 22 - Prob. 154CWPCh. 22 - Prob. 155CWPCh. 22 - Prob. 156CWPCh. 22 - Prob. 157CPCh. 22 - Estimate H for the following reactions using bond...Ch. 22 - Prob. 159CPCh. 22 - Prob. 160CPCh. 22 - Prob. 161CPCh. 22 - Consider a sample of a hydrocarbon at 0.959 atm...Ch. 22 - Prob. 163CPCh. 22 - Prob. 164CPCh. 22 - Prob. 165CPCh. 22 - Prob. 166CPCh. 22 - Prob. 167CPCh. 22 - Prob. 168CPCh. 22 - Prob. 169CPCh. 22 - Alcohols are very useful starting materials for...Ch. 22 - A chemical breathalyzer test works because ethanol...Ch. 22 - Estradiol is a female hormone with the following...Ch. 22 - Prob. 173IPCh. 22 - Prob. 174IPCh. 22 - Prob. 175MPCh. 22 - Prob. 176MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B Bond A Bond C a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest Bond Strongest Bond b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. c. (5pts) Use principles discussed in lecture, supported by relevant structures, to succinctly explain the why your part b (i) radical is more stable than your part b(ii) radical. Written explanation can be no more than one-two succinct sentence(s)!arrow_forward. 3°C with TH 12. (10pts total) Provide the major product for each reaction depicted below. If no reaction occurs write NR. Assume heat dissipation is carefully controlled in the fluorine reaction. 3H 24 total (30) 24 21 2h • 6H total ● 8H total 34 래 Br2 hv major product will be most Substituted 12 hv Br NR I too weak of a participate in P-1 F₂ hv Statistically most favored product will be major = most subst = thermo favored hydrogen atom abstractor to LL Farrow_forwardFive chemistry project topic that does not involve practicalarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardQ2. Consider the hydrogenation of ethylene C2H4 + H2 = C2H6 The heats of combustion and molar entropies for the three gases at 298 K are given by: C2H4 C2H6 H2 AH comb/kJ mol¹ -1395 -1550 -243 Sº / J K¹ mol-1 220.7 230.4 131.1 The average heat capacity change, ACP, for the reaction over the temperature range 298-1000 K is 10.9 J K¹ mol¹. Using these data, determine: (a) the standard enthalpy change at 800 K (b) the standard entropy change at 800 K (c) the equilibrium constant at 800 K.arrow_forward13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B Bond A Bond C a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest Bond Strongest Bond b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. c. (5pts) Use principles discussed in lecture, supported by relevant structures, to succinctly explain the why your part b (i) radical is more stable than your part b(ii) radical. Written explanation can be no more than one-two succinct sentence(s)! Googlearrow_forward
- Print Last Name, First Name Initial Statifically more chances to abstract one of these 6H 11. (10pts total) Consider the radical chlorination of 1,3-diethylcyclohexane depicted below. 4 4th total • 6H total 래 • 4H total 21 total ZH 2H Statistical H < 3° C-H weakest - product abstraction here bund leads to thermo favored a) (6pts) How many unique mono-chlorinated products can be formed and what are the structures for the thermodynamically and statistically favored products? Product 6 Number of Unique Mono-Chlorinated Products Thermodynamically Favored Product Statistically Favored Product b) (4pts) Draw the arrow pushing mechanism for the FIRST propagation step (p-1) for the formation of the thermodynamically favored product. Only draw the p-1 step. You do not need to include lone pairs of electrons. No enthalpy calculation necessary H H-Cl Waterfoxarrow_forward10. (5pts) Provide the complete arrow pushing mechanism for the chemical transformation → depicted below Use proper curved arrow notation that explicitly illustrates all bonds being broken, and all bonds formed in the transformation. Also, be sure to include all lone pairs and formal charges on all atoms involved in the flow of electrons. CH3O II HA H CH3O-H H ①arrow_forwardDo the Lone Pairs get added bc its valence e's are a total of 6 for oxygen and that completes it or due to other reasons. How do we know the particular indication of such.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY