
Concept explainers
a)
Interpretation:
The straight-chain structure of the given molecule should be stated.
Concept Introduction:
The systematic structure determination of organic compounds is given by IUPAC. The name of the organic compound is determined from the structure that is by analyzing the position of groups substituted on it.
a)
Answer to Problem 30A
The straight-chain structure of the monosaccharide glucoseis:
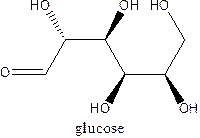
Explanation of Solution
Glucose is a carbohydrate which is a hexose. The straight-chain structurecontains 6 carbon atoms,
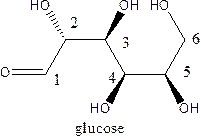
b)
Interpretation:
The straight-chain structure of the given molecule should be stated.
Concept Introduction:
The systematic structure determination of organic compounds is given by IUPAC. The name of the organic compound is determined from the structure that is by analyzing the position of groups substituted on it.
b)
Answer to Problem 30A
The straight-chain structure of the monosaccharide ribose is:
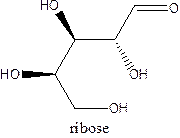
Explanation of Solution
Ribulose is a carbohydrate that is pentose. A straight-chain structure of 5 carbon atomcontains
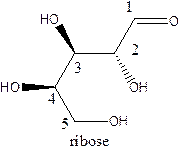
c)
Interpretation:
The straight-chain structure of the given molecule should be stated.
Concept Introduction:
The systematic structure determination of organic compounds is given by IUPAC. The name of the organic compound is determined from the structure that is by analyzing the position of groups substituted on it.
c)
Answer to Problem 30A
The straight-chain structure of the monosaccharide ribulose is:
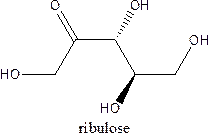
Explanation of Solution
Ribulose is a carbohydrate that is pentose. A straight-chain structure of 5 carbon atomcontains ketone at 2nd position and then 1,3,4, and 5 position contains OH group.The third position carbon is an anomeric carbon center which has a different configuration than the other OH.
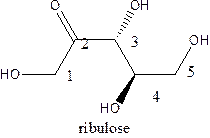
d)
Interpretation:
The straight-chain structure of the given molecule should be stated.
Concept Introduction:
The systematic structure determination of organic compounds is given by IUPAC. The name of the organic compound is determined from the structure that is by analyzing the position of groups substituted on it.
d)
Answer to Problem 30A
The straight-chain structure of the monosaccharide galactose is:
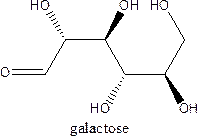
Explanation of Solution
Galactose is a carbohydratethat is a hexose. A straight-chain structure contains 6 carbon atoms, aldehyde at 1st position and then 2,3,4,5 and 6 position contains OH group. The second position carbon and 4th position carbon have the same configuration while 3rd and 5th carbon atom hasthe same configuration
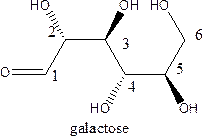
Chapter 21 Solutions
World of Chemistry
- (f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





