
Concept explainers
(a)
Interpretation: The systematic name of the given substituted alkane needs to be determined.
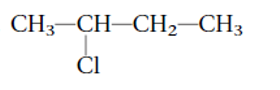
Concept Introduction:
The IUPAC rules are purposed to names different alkanes and alkyl halides.
(a)
Answer to Problem 62A
2-bromobutane
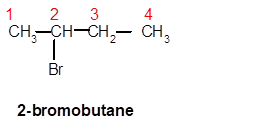
Explanation of Solution
According to IUPAC rule:
- Root word will be determined on the basis of number of C atoms in the longest chain.
- The primary suffix must be determined on the basis of covalent bonds between carbon atoms of the parent chain. They can be −ane, -ene and −yne.
- The secondary suffix indicates the presence of
functional group in the molecule. - The prefix in the IUPAC name indicates the presence of side chain or certain groups like halogens.
Thus, in the given alkane, the name of compound must be 2-bromobutaneas the longest chain contains 4 C atoms with all single covalent bonds. There is one Br group bonded at C-2 position.
(b)
Interpretation: The systematic name of the given substituted alkane needs to be determined.
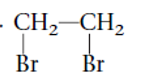
Concept Introduction: Alkanes are the saturated hydrocarbons which are mainly composed of C and H atoms. The carbon atoms are bonded in straight chain or branched chain. Alkyl halides are the halogens derivatives of alkanes. They have certain heteroatoms as Cl, I, Br or F along with C and H atoms.
The IUPAC rules are purposed to names different alkanes and alkyl halides.
(b)
Answer to Problem 62A
1,2-dibromoethane
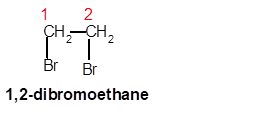
Explanation of Solution
According to IUPAC rule:
- Root word will be determined on the basis of number of C atoms in the longest chain.
- The primary suffix must be determined on the basis of covalent bonds between carbon atoms of the parent chain. They can be −ane, -ene and −yne.
- The secondary suffix indicates the presence of functional group in the molecule.
- The prefix in the IUPAC name indicates the presence of side chain or certain groups like halogens.
Thus in the given alkane, the name of compound must be 1,2-dibromoethane as the longest chain contains 2 C atoms with all single covalent bond and there are two Br groups at C-1 and C-2 positions.
(c)
Interpretation: The systematic name of the given substituted alkane needs to be determined.
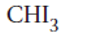
Concept Introduction: Alkanes are the saturated hydrocarbons which are mainly composed of C and H atoms. The carbon atoms are bonded in straight chain or branched chain. Alkyl halides are the halogens derivatives of alkanes. They have certain heteroatoms as Cl, I, Br or F along with C and H atoms.
The IUPAC rules are purposed to names different alkanes and alkyl halides.
(c)
Answer to Problem 62A
Triiodomethane
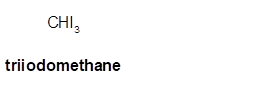
Explanation of Solution
According to IUPAC rule:
- Root word will be determined on the basis of number of C atoms in the longest chain.
- The primary suffix must be determined on the basis of covalent bonds between carbon atoms of the parent chain. They can be −ane, -ene and −yne.
- The secondary suffix indicates the presence of functional group in the molecule.
- The prefix in the IUPAC name indicates the presence of side chain or certain groups like halogens.
Thus in the given alkane, the name of compound must be triiodomethane as the longest chain contains 1 C atom with 3I groups on same C atom.
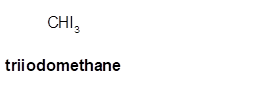
(d)
Interpretation: The systematic name of the given substituted alkane needs to be determined.
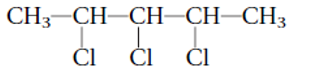
Concept Introduction: Alkanes are the saturated hydrocarbons which are mainly composed of C and H atoms. The carbon atoms are bonded in straight chain or branched chain. Alkyl halides are the halogens derivatives of alkanes. They have certain heteroatoms as Cl, I, Br or F along with C and H atoms.
The IUPAC rules are purposed to names different alkanes and alkyl halides.
(d)
Answer to Problem 62A
2,3,4-tribromohexane
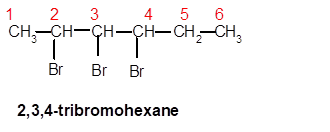
Explanation of Solution
According to IUPAC rule:
- Root word will be determined on the basis of number of C atoms in the longest chain.
- The primary suffix must be determined on the basis of covalent bonds between carbon atoms of the parent chain. They can be −ane, -ene and −yne.
- The secondary suffix indicates the presence of functional group in the molecule.
- The prefix in the IUPAC name indicates the presence of side chain or certain groups like halogens.
Thus in the given alkane, the name of compound must be 2,3,4-tribromohexane as the longest chain contains 6 C atom with 3 Br groups at C-2,3,and C-4 atoms.
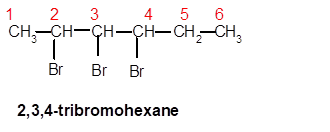
(e)
Interpretation: The systematic name of the given substitute alkane needs to be determined.
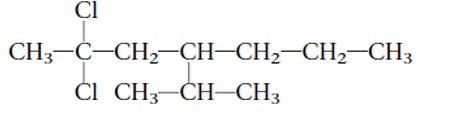
Concept Introduction: Alkanes are the saturated hydrocarbons which are mainly composed of C and H atoms. The carbon atoms are bonded in straight chain or branched chain. Alkyl halides are the halogens derivatives of alkanes. They have certain heteroatoms as Cl, I, Br or F along with C and H atoms.
The IUPAC rules are purposed to names different alkanes and alkyl halides.
(e)
Answer to Problem 62A
2,2-dichloro-4-isopropylheptane
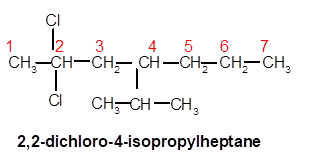
Explanation of Solution
According to IUPAC rule:
- Root word will be determined on the basis of number of C atoms in the longest chain.
- The primary suffix must be determined on the basis of covalent bonds between carbon atoms of the parent chain. They can be −ane, -ene and −yne.
- The secondary suffix indicates the presence of functional group in the molecule.
- The prefix in the IUPAC name indicates the presence of side chain or certain groups like halogens.
Thus in the given alkane, the name of compound must be 2,2-dichloro-4-isopropylheptaneas the longest chain contains 7 C atom with 2Cl groups at C-2 and one isopropyl group at C-4.
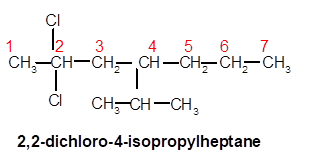
Chapter 20 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





